In essence, calcination is a purification and transformation process driven by heat. It is a specific type of heat treatment applied to solid materials, where they are heated to a high temperature but below their melting point. This is performed in the absence or with a very limited supply of air, which is a critical detail that distinguishes it from other thermal processes. The primary goals are to induce thermal decomposition, drive off volatile substances like water or carbon dioxide, or trigger a change in the material's crystal structure.
Calcination is not simply about heating a material; it is a precise thermal process designed to change a material's chemical composition or physical structure without melting it. The key is controlling the temperature and atmosphere to achieve a specific outcome, like decomposition or purification.

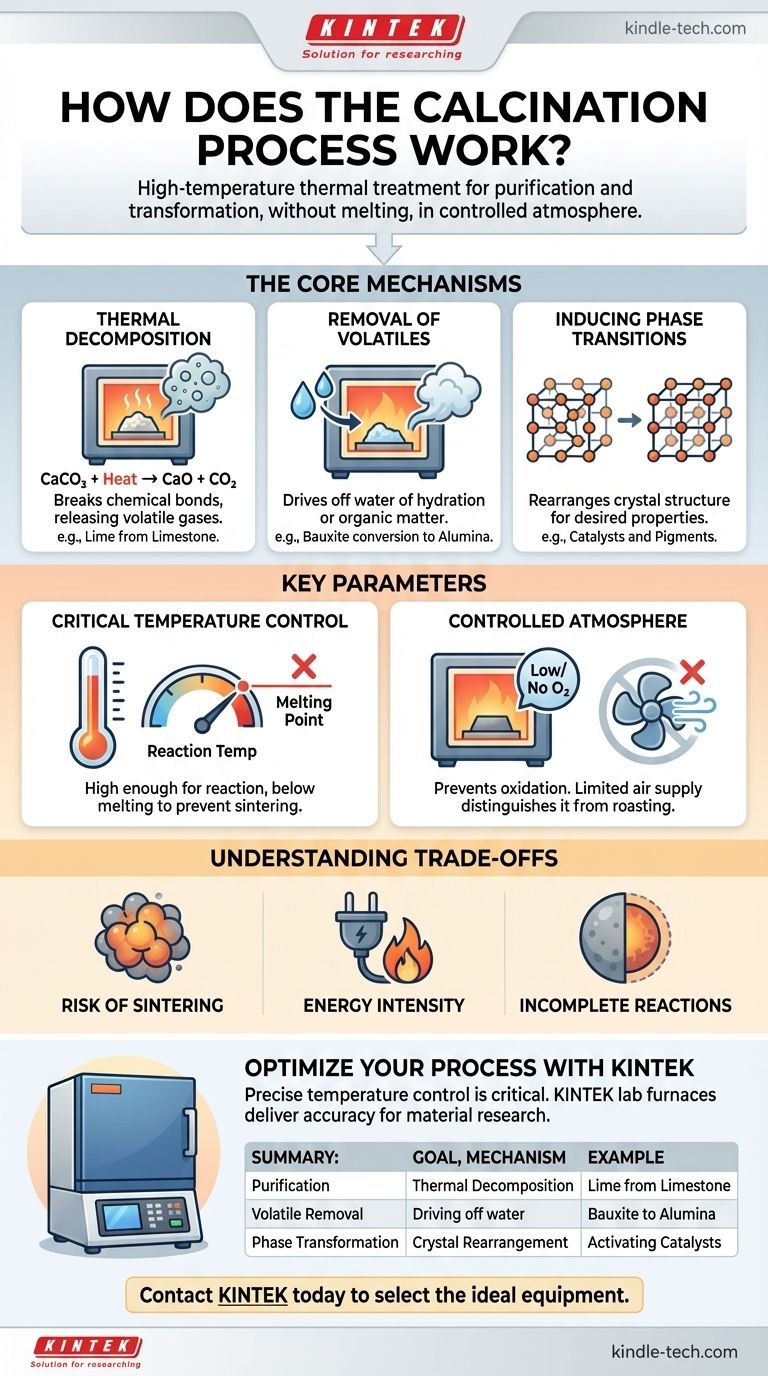
The Core Mechanisms of Calcination
Calcination achieves its results through several distinct physical and chemical changes. The specific goal of the process determines which of these mechanisms is most important.
Thermal Decomposition
This is the most common objective of calcination. The process applies enough thermal energy to break chemical bonds within a compound, decomposing it into simpler substances.
A classic industrial example is the production of lime (calcium oxide) from limestone (calcium carbonate). When heated, the carbonate decomposes, releasing carbon dioxide gas.
CaCO₃ (solid) + Heat → CaO (solid) + CO₂ (gas)
This resulting calcium oxide is a fundamental component in the manufacturing of cement and steel.
Removal of Volatiles
Many raw minerals contain water, either physically absorbed or chemically bound within their crystal structure (known as water of hydration). Calcination provides the energy to drive this water off as steam.
For instance, the conversion of bauxite into alumina for aluminum production involves calcination to remove its water of hydration. Similarly, this process is used to remove volatile organic matter from various materials.
Inducing Phase Transitions
Sometimes, the goal is not to change the chemical composition but to alter the material's physical properties. Heating a material can cause its atoms to rearrange into a different, often more stable or functionally useful, crystal structure.
This is common in the production of catalysts and ceramic pigments, where a specific crystalline phase possesses the desired catalytic activity or color.
Key Parameters That Define the Process
Successfully calcining a material requires precise control over several variables. These parameters dictate the efficiency and outcome of the entire process.
The Critical Role of Temperature
The calcination temperature must be carefully controlled. It needs to be high enough to supply the activation energy for the desired reaction (e.g., decomposition) but must remain strictly below the material's melting point.
If the temperature is too low, the reaction will be incomplete. If it is too high, the material may melt or sinter (fuse together), which is typically an undesirable outcome.
The Controlled Atmosphere
Calcination is defined by its low-oxygen or oxygen-free environment. This is crucial for preventing oxidation, which is an entirely different chemical reaction.
Processes that heat materials in the presence of abundant air to deliberately cause oxidation are known as roasting, not calcination. This distinction is fundamental in metallurgy and materials science.
Understanding the Trade-offs and Limitations
While powerful, calcination is not without its challenges. Understanding its limitations is key to applying it effectively.
Risk of Sintering
Poor temperature control is the primary risk. If the temperature gets too close to the material's melting point, individual particles can begin to fuse together. This sintering process reduces the surface area and can ruin the desired properties of the final product.
Energy Intensity
Maintaining the high temperatures required for calcination (often 800-1000°C or higher) demands a significant amount of energy. This makes it a costly process with a notable environmental footprint, especially for reactions like lime production that release large volumes of CO₂.
Incomplete Reactions
Achieving a 100% complete reaction can be difficult. If the material is not heated for a sufficient duration, or if heat transfer is poor due to large particle sizes, some of the original, un-decomposed material may remain, compromising the purity of the final product.
Making the Right Choice for Your Goal
The specific parameters of your calcination process depend entirely on your desired end product.
- If your primary focus is producing an oxide from a carbonate: You must ensure the temperature is high enough to drive off all CO₂ but low enough to prevent sintering of the final oxide product.
- If your primary focus is removing moisture: A lower temperature profile may be sufficient, focusing on driving off water without triggering unwanted chemical decomposition.
- If your primary focus is achieving a specific crystal phase: Precise temperature control and hold time are paramount, as phase transitions occur within specific temperature windows.
Ultimately, mastering calcination lies in understanding it as a tool to precisely engineer the final properties of a material through controlled thermal energy.
Summary Table:
| Goal of Calcination | Key Mechanism | Example Application |
|---|---|---|
| Purification / Decomposition | Thermal decomposition of compounds (e.g., carbonates). | Producing lime (CaO) from limestone (CaCO₃). |
| Removal of Volatiles | Driving off water (hydration) or other volatile substances. | Converting bauxite to alumina for aluminum production. |
| Phase Transformation | Rearranging crystal structure to alter material properties. | Activating catalysts or developing ceramic pigments. |
Ready to Optimize Your Calcination Process?
Precise temperature control is critical for successful calcination. KINTEK specializes in high-performance lab furnaces that deliver the accuracy and reliability your laboratory needs for material purification, decomposition, and phase transformation research.
Contact KINTEK today to discuss your specific application. Our experts will help you select the ideal equipment to achieve your material science goals.
Visual Guide
Related Products
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What 5 safety precautions should be taken when heating anything in the lab? Essential Rules for Lab Safety
- How do you prepare samples for IR? A Guide to Solid, Liquid, and Gas Sample Prep
- What is a muffle furnace used for in microbiology? Essential for Depyrogenation and Ashing
- What are the environmental impacts of metal processing? A Guide to Sustainability and Solutions
- What are the stages of melting metal? Mastering the 3-Step Process from Solid to Liquid



















