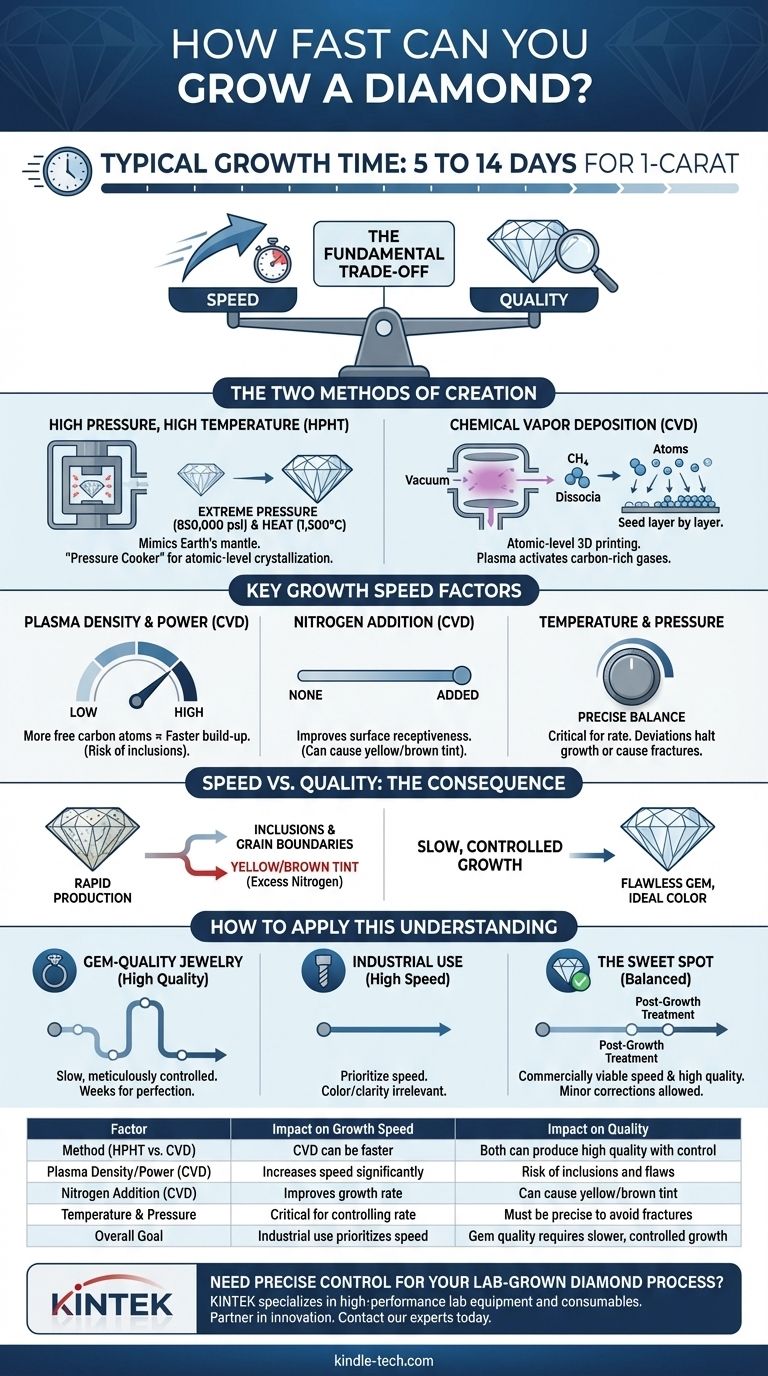
In practice, growing a 1-carat lab diamond can take anywhere from five to fourteen days, but this is not a fixed number. The speed of diamond growth is a highly variable process, deliberately controlled by producers who must constantly balance the rate of creation against the final quality of the stone. The specific method used and the desired size and clarity are the primary factors that dictate the total time required.
The core issue is not simply how fast a diamond can be grown, but the fundamental trade-off between speed and quality. Accelerating the growth process almost always introduces imperfections, forcing manufacturers to choose between rapid production and creating a flawless gem.

The Two Methods of Diamond Creation
To understand growth speed, you must first understand the two dominant methods for creating lab-grown diamonds: High Pressure, High Temperature (HPHT) and Chemical Vapor Deposition (CVD).
The High Pressure, High Temperature (HPHT) Method
HPHT mimics the natural diamond-forming process of the Earth's mantle. A small diamond "seed" is placed in a chamber with a source of pure carbon.
This chamber is then subjected to immense pressure (over 850,000 psi) and extreme heat (around 1,500°C or 2,700°F).
Under these conditions, the carbon melts and begins to crystallize onto the diamond seed, growing a larger, single-crystal diamond. This is conceptually similar to a pressure cooker, but operating at an astronomical scale of heat and force.
The Chemical Vapor Deposition (CVD) Method
The CVD process is more akin to atomic-level 3D printing. A thin diamond seed plate is placed inside a vacuum chamber.
The chamber is filled with carbon-rich gases, such as methane. These gases are then heated to a plasma state using microwaves, causing the carbon atoms to separate from their gas molecules.
These individual carbon atoms then "rain down" and deposit onto the diamond seed, building up the diamond layer by layer.
Key Factors That Control Growth Speed
Producers can manipulate several variables to either speed up or slow down the growth process, each with significant consequences.
Plasma Density and Power (CVD)
As noted in research, increasing the density of the plasma in a CVD reactor directly increases the growth rate. This is achieved by raising the pressure inside the chamber or increasing the microwave power.
A denser plasma means more free carbon atoms are available to deposit onto the seed at any given moment, accelerating the build-up.
The Role of Nitrogen Gas (CVD)
The strategic addition of small amounts of nitrogen gas into the CVD chamber is another well-known technique to improve the growth rate.
Nitrogen helps create specific growth patterns on the diamond's surface that are more receptive to carbon atoms, effectively speeding up how quickly they can bond to the crystal lattice.
Temperature and Pressure
In both HPHT and CVD, temperature and pressure are the master controls. Fine-tuning these parameters is essential. A slight deviation can halt growth entirely or, if pushed too high too fast, can cause the diamond crystal to fracture or develop significant internal flaws.
Understanding the Trade-off: Speed vs. Quality
The pursuit of speed is never without consequence. Pushing the growth process to its limits invariably compromises the final quality of the diamond.
Inclusions and Grain Boundaries
When growth is too rapid, the crystal lattice doesn't have time to form perfectly. This can lead to other non-carbon elements getting trapped within the diamond, creating inclusions.
In extreme cases, multiple small crystals can start to form instead of one large one, creating grain boundaries that ruin the gem's integrity.
Impact on Color and Clarity
The use of nitrogen to accelerate CVD growth is a perfect example of this trade-off. While it speeds up the process, excess nitrogen atoms can become trapped in the diamond's crystal structure.
These trapped nitrogen atoms absorb blue light, giving the diamond an undesirable yellow or brownish tint. Producers must find the perfect balance to gain speed without sacrificing color.
How to Apply This Understanding
The optimal growth rate is not a universal figure; it is determined entirely by the intended purpose of the final product.
- If the primary focus is maximum quality: The growth process must be slow and meticulously controlled, often taking several weeks for a single large gem, minimizing any risk of inclusions or color impurities.
- If the primary focus is industrial use (e.g., abrasives, drill bits): Speed is prioritized over quality. These diamonds can be grown very quickly, as color, clarity, and internal flaws are irrelevant to their function.
- If the primary focus is gem-quality jewelry: Producers find a "sweet spot" that balances a commercially viable growth speed with a high-quality result, often relying on post-growth treatments to correct minor color issues.
Ultimately, growing a diamond is a precise act of atomic engineering where time itself is the most critical variable to control.
Summary Table:
| Factor | Impact on Growth Speed | Impact on Quality |
|---|---|---|
| Method (HPHT vs. CVD) | CVD can be faster | Both can produce high quality with control |
| Plasma Density/Power (CVD) | Increases speed significantly | Risk of inclusions and flaws |
| Nitrogen Addition (CVD) | Improves growth rate | Can cause yellow/brown tint |
| Temperature & Pressure | Critical for controlling rate | Must be precise to avoid fractures |
| Overall Goal | Industrial use prioritizes speed | Gem quality requires slower, controlled growth |
Need Precise Control for Your Lab-Grown Diamond Process?
The journey from a diamond seed to a flawless gem requires not just advanced technology, but a deep understanding of the intricate balance between speed and quality. At KINTEK, we specialize in providing the high-performance lab equipment and consumables essential for this precise atomic engineering.
Whether you are optimizing a CVD reactor's plasma parameters or calibrating an HPHT press, our expertise supports your mission to produce superior diamonds efficiently. We serve laboratories focused on both industrial applications and high-end gem production.
Let KINTEK be your partner in innovation. Contact our experts today to discuss how our solutions can enhance your diamond growth process, improve your yield, and help you achieve the perfect balance for your specific needs.
Visual Guide
Related Products
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- CVD Diamond Domes for Industrial and Scientific Applications
- Laboratory CVD Boron Doped Diamond Materials
People Also Ask
- What is diamond coating film? A Thin Layer of Diamond for Extreme Performance
- What are the industrial uses of CVD diamond? Unlock Extreme Performance in Your Applications
- What are diamond films used for? Enhancing Tools, Electronics, and Implants with Diamond Surfaces
- What do you need to grow lab diamonds? Carbon, Seed, and Immense Energy Explained
- What is the process for CVD diamond? Building a Diamond Atom by Atom
- How does Microwave Plasma Chemical Vapour Deposition (MPCVD) work? Your Guide to High-Purity Diamond Film Growth
- Is there certification for lab-grown diamonds? Get Independent Verification for Your Purchase
- How is a CVD diamond formed? A Guide to Lab-Grown Diamond Creation



















