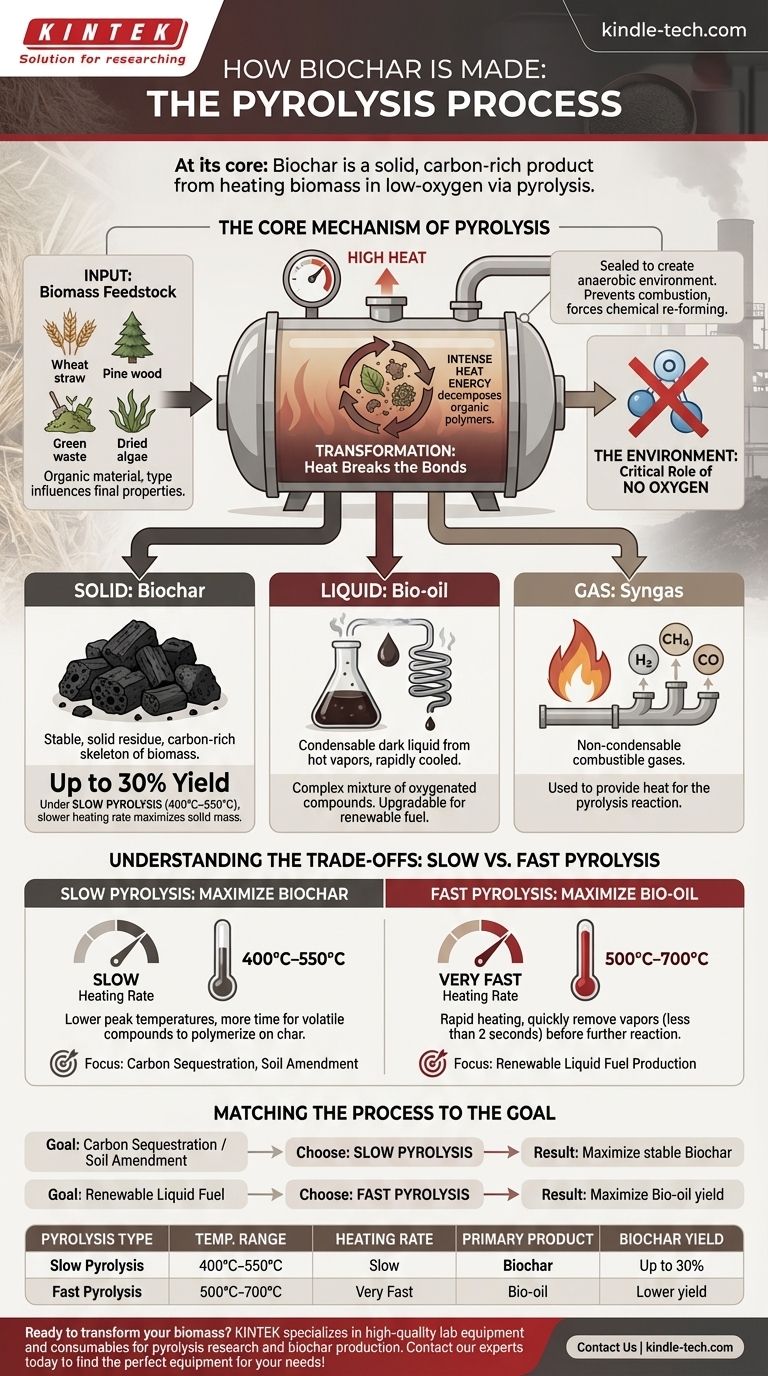
At its core, biochar is the solid, carbon-rich product created by heating biomass in a low-oxygen environment through a process called pyrolysis. This thermal decomposition breaks down the complex organic material into a stable, charcoal-like substance, along with a liquid (bio-oil) and a gas (syngas). The absence of oxygen is critical, as it prevents the biomass from combusting and instead forces it to chemically re-form.
The key to understanding biochar production is realizing that pyrolysis is not a single method, but a tunable process. By controlling variables like temperature and heating rate, producers can deliberately optimize the output to yield more biochar, more bio-oil, or more gas depending on their primary goal.

The Core Mechanism of Pyrolysis
Pyrolysis is a fundamental chemical transformation driven by heat in an inert atmosphere. Understanding this process is key to understanding biochar itself.
The Input: Biomass Feedstock
The process begins with biomass, which is any organic material. The type of feedstock influences the final properties of the biochar.
Common feedstocks include agricultural waste like wheat straw, woody materials like pine wood, municipal green waste, and even specialized inputs like dried algae.
The Environment: The Critical Role of No Oxygen
Pyrolysis equipment, often called a reactor or kiln, is sealed to create an oxygen-free (or anaerobic) environment.
This is the most important factor distinguishing pyrolysis from burning. Without oxygen, the biomass cannot combust into ash and smoke. Instead, the heat forces the chemical bonds within the material to break.
The Transformation: Heat Breaks the Bonds
Inside the reactor, the biomass is heated to high temperatures. The intense heat energy causes the large organic polymers in the biomass (like cellulose and lignin) to decompose into smaller, volatile molecules and a stable, solid carbon structure.
The Three Products of Pyrolysis
The decomposition of biomass via pyrolysis results in three distinct product streams: a solid, a liquid, and a gas.
Solid: Biochar
This is the stable, solid residue left after the volatile components have been driven off. It is highly rich in carbon and forms the "skeleton" of the original biomass.
Under conditions optimized for biochar, known as slow pyrolysis, the yield can be up to 30% of the initial dry feedstock weight.
Liquid: Bio-oil
As the biomass heats, it releases vapors. When these hot vapors are captured and rapidly cooled, they condense into a dark liquid known as bio-oil or pyrolysis oil.
This bio-oil is a complex mixture of oxygenated organic compounds and can be upgraded for use as a renewable fuel.
Gas: Syngas
Not all the gases released during pyrolysis will condense into a liquid. This non-condensable stream is called synthesis gas, or syngas.
It is a mixture of combustible gases like hydrogen, methane, and carbon monoxide, and it is often captured and used to provide the heat needed to sustain the pyrolysis reaction itself.
Understanding the Trade-offs: Slow vs. Fast Pyrolysis
The final yields of biochar, bio-oil, and syngas are not fixed. They are determined by the specific pyrolysis conditions, primarily the heating rate and temperature.
Slow Pyrolysis: Maximizing Biochar
To maximize the biochar yield, producers use slow pyrolysis. This involves heating the biomass at a slower rate to lower peak temperatures (typically 400°C–550°C).
These conditions give the volatile compounds more time to interact and polymerize on the surface of the evolving char, increasing the final solid mass.
Fast Pyrolysis: Maximizing Bio-oil
To maximize the liquid bio-oil yield, producers use fast pyrolysis. This process involves heating the biomass very rapidly to higher temperatures (500°C–700°C).
The goal is to break down the biomass and get the resulting vapors out of the hot reactor as quickly as possible (in less than 2 seconds) before they can further react, maximizing the amount of condensable liquid.
Matching the Process to the Goal
Choosing the right pyrolysis method depends entirely on the desired end product. The process is a set of levers that can be pulled to achieve a specific outcome.
- If your primary focus is carbon sequestration or soil amendment: Slow pyrolysis is the correct path, as it is engineered to maximize the production of stable, solid biochar.
- If your primary focus is producing renewable liquid fuel: Fast pyrolysis is the superior choice, as its conditions are optimized to generate the highest possible yield of bio-oil.
By understanding these fundamental levers, you can select or design a pyrolysis process that precisely matches your desired outcome.
Summary Table:
| Pyrolysis Type | Temperature Range | Heating Rate | Primary Product | Biochar Yield |
|---|---|---|---|---|
| Slow Pyrolysis | 400°C–550°C | Slow | Biochar | Up to 30% |
| Fast Pyrolysis | 500°C–700°C | Very Fast | Bio-oil | Lower yield |
Ready to transform your biomass into valuable products? KINTEK specializes in high-quality lab equipment and consumables for pyrolysis research and biochar production. Whether you're focused on soil amendment, carbon sequestration, or renewable energy, our solutions help you optimize your process for maximum efficiency and yield. Contact our experts today to find the perfect equipment for your laboratory needs!
Visual Guide
Related Products
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vertical Laboratory Tube Furnace
- Graphite Vacuum Continuous Graphitization Furnace
People Also Ask
- What is a fluidized bed chemical reaction? A Guide to Superior Heat Transfer & Continuous Processing
- What is the process of catalytic pyrolysis? Upgrade Biomass and Plastic Waste into High-Quality Fuel
- What are the different types of rotary kiln incinerators? Find the Right Design for Your Waste Stream
- What are the disadvantages of rotary kiln incinerator? High Costs and Operational Complexities
- How does a rotary calciner work? Achieve Uniform Thermal Processing for Bulk Solids
- What are the heating sources for pyrolysis? Direct vs. Indirect Methods for Optimal Product Yield
- What is a calciner kiln? The High-Temperature Reactor for Material Transformation
- What technical requirements must high-temperature industrial kilns meet for the chlorination roasting of quartz sand?



















