In short, pyrolysis produces hydrogen by heating a hydrocarbon source, such as natural gas (methane), to very high temperatures in an oxygen-free environment. This intense heat breaks the molecular bonds of the methane, causing it to decompose directly into its constituent elements: hydrogen gas and solid carbon. This process avoids the chemical reaction with oxygen or water that creates CO2 in other methods.
Methane pyrolysis is a fundamentally different approach to hydrogen production. Instead of creating CO₂ as a byproduct, it physically separates the hydrogen and carbon atoms from natural gas, offering a less energy-intensive path to low-carbon hydrogen, provided the solid carbon is properly managed.

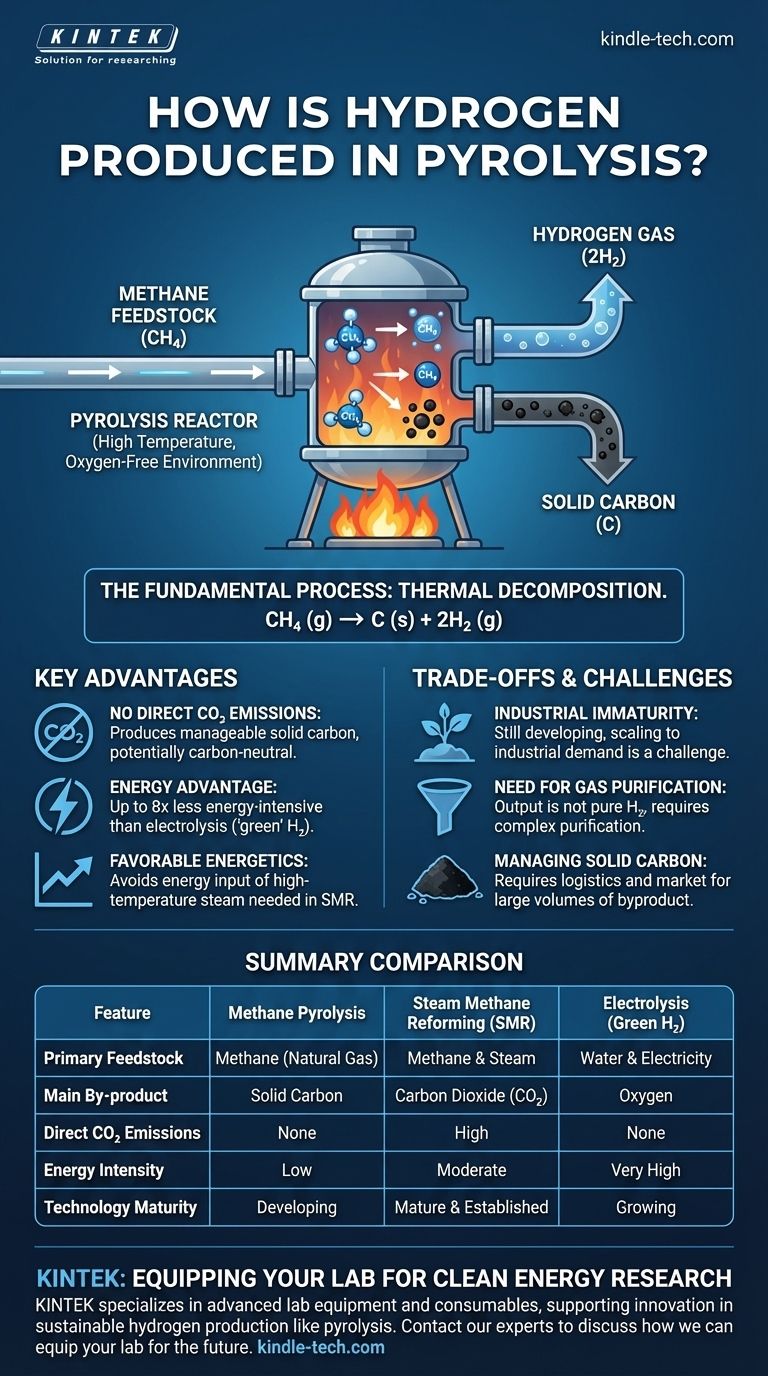
The Fundamental Process: Thermal Decomposition
Pyrolysis is a simple yet powerful method of thermal decomposition. Understanding its core mechanism reveals why it is gaining attention as a clean hydrogen pathway.
How It Works
The process involves placing a feedstock, primarily methane (CH₄), into a reactor heated to high temperatures.
Crucially, this is done in an inert atmosphere without oxygen. The absence of oxygen prevents combustion and instead forces the methane molecules to crack, or decompose, under the thermal energy.
The Chemical Reaction
The overall chemical equation for methane pyrolysis is straightforward: CH₄ (g) → C (s) + 2H₂ (g).
This shows that one molecule of methane gas is converted into one atom of solid carbon and two molecules of hydrogen gas. There are no other outputs in the core reaction.
Pyrolysis vs. Conventional Hydrogen Production
To understand the significance of pyrolysis, it's essential to compare it to the dominant methods used today: steam-methane reforming (SMR) and electrolysis ("green" hydrogen).
Advantage 1: No Direct CO₂ Emissions
The primary drawback of steam reforming, the current industry standard, is that it generates a significant amount of carbon dioxide.
Pyrolysis, by contrast, produces carbon in a solid, manageable form. If this solid carbon is sequestered or used in materials like asphalt or batteries, the entire process can be considered carbon-neutral or even carbon-negative.
Advantage 2: A Major Energy Advantage
Producing "green" hydrogen via electrolysis requires vast amounts of electricity to split water molecules.
Methane pyrolysis is far less energy-intensive. Some methods can produce hydrogen using up to eight times less energy than electrolysis, dramatically lowering the operational cost and electrical grid burden.
Advantage 3: Favorable Energetics
Compared to steam reforming, methane pyrolysis is also more energetically favorable. It avoids the significant energy input required to produce the high-temperature steam needed for the SMR process.
Understanding the Trade-offs and Challenges
While promising, pyrolysis is not a perfect solution. Acknowledging its current limitations is critical for a balanced assessment.
Industrial Immaturity
Steam reforming is a mature, state-of-the-art technology that has been optimized for decades.
Methane pyrolysis has not yet been commercialized on a large scale. The technology is still developing, and scaling it to meet industrial hydrogen demand remains a significant engineering challenge.
The Need for Gas Purification
The output from a pyrolysis reactor is not pure hydrogen. It contains unreacted methane and other hydrocarbon by-products.
To produce high-purity hydrogen suitable for applications like fuel cells or the petrochemical industry, the gas mixture must undergo a costly and complex purification process.
Managing the Solid Carbon By-product
The process creates a massive amount of solid carbon. While this can be a valuable co-product, developing a market and the logistics to handle millions of tons of carbon is a challenge that must be solved for pyrolysis to be truly sustainable at scale.
Making the Right Choice for Your Goal
Evaluating hydrogen production methods depends entirely on your primary objective.
- If your primary focus is avoiding CO₂ emissions with mature technology: Steam reforming paired with carbon capture and storage (CCS) is the most established route for "blue" hydrogen today.
- If your primary focus is energy efficiency and a low carbon footprint: Methane pyrolysis offers a compelling advantage over both electrolysis and steam reforming, provided a plan exists for the solid carbon.
- If your primary focus is immediate, large-scale production: Steam reforming remains the dominant, proven, and most cost-effective technology, despite its environmental drawbacks.
Ultimately, methane pyrolysis shifts the hydrogen challenge from managing a gaseous CO₂ emission to managing a solid carbon by-product, offering a promising but still-developing new frontier.
Summary Table:
| Feature | Methane Pyrolysis | Steam Methane Reforming (SMR) | Electrolysis (Green H₂) |
|---|---|---|---|
| Primary Feedstock | Methane (Natural Gas) | Methane & Steam | Water & Electricity |
| Main By-product | Solid Carbon | Carbon Dioxide (CO₂) | Oxygen |
| Direct CO₂ Emissions | None | High | None |
| Energy Intensity | Low | Moderate | Very High |
| Technology Maturity | Developing | Mature & Established | Growing |
Ready to explore clean hydrogen solutions for your laboratory or industrial process?
KINTEK specializes in advanced lab equipment and consumables, providing the tools and expertise needed to research and develop next-generation energy technologies like pyrolysis. Whether you're optimizing reactor designs or analyzing carbon by-products, our high-quality products support innovation in sustainable hydrogen production.
Contact our experts today to discuss how KINTEK can equip your lab for the future of clean energy.
Visual Guide
Related Products
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Laboratory High Pressure Vacuum Tube Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- How to clean a tube furnace? A Step-by-Step Guide for Safe and Effective Maintenance
- What is the temperature of a tube furnace? A Guide to High-Temp Heating Elements & Control
- What precautions should be taken when using a tube furnace? Ensure Safe, Effective High-Temperature Processing
- How does a high-temperature tube furnace facilitate the phase transformation of alumina products? Master Thermal Control
- What is the technical value of using a quartz tube reaction chamber for static corrosion testing? Achieve Precision.



















