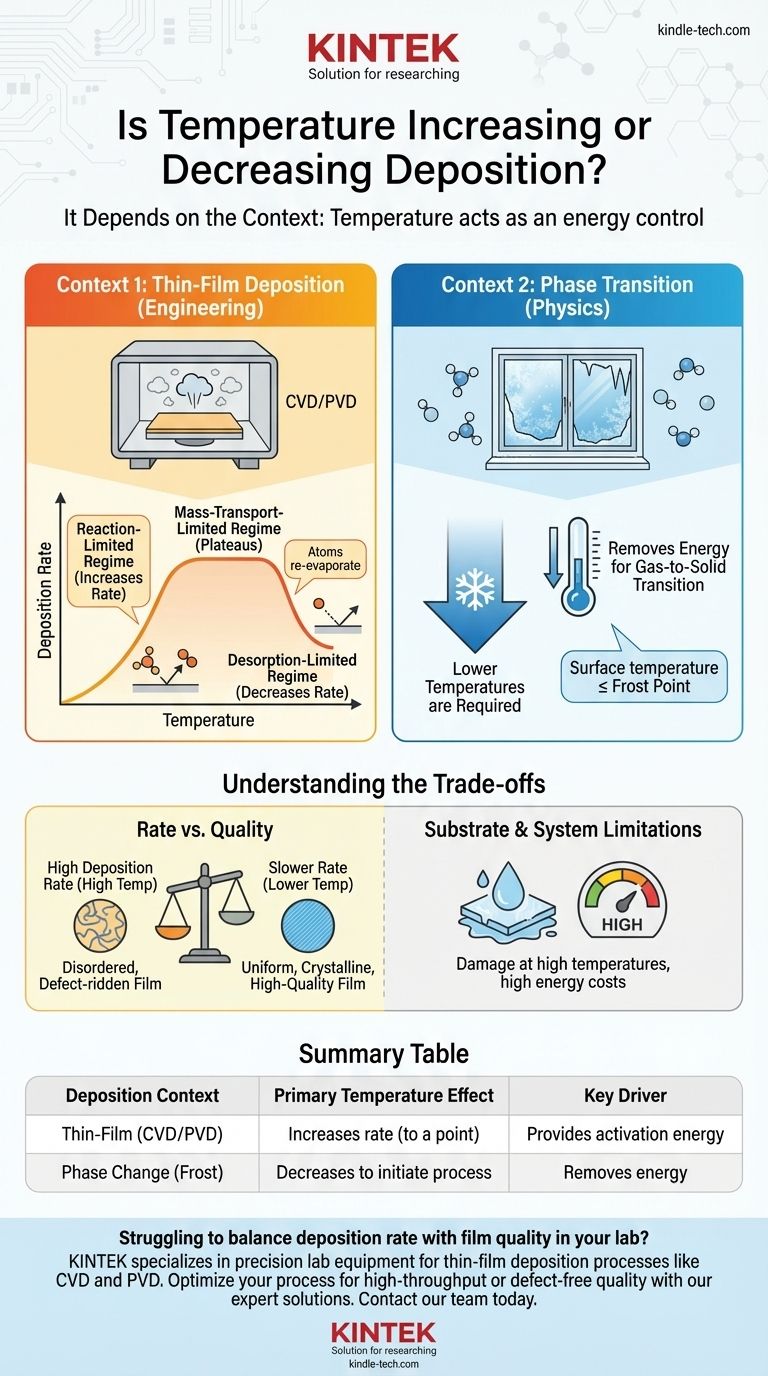
In most technical applications, increasing temperature increases the rate of deposition, but only up to a critical point. The relationship is not linear; for the natural phase change of a gas to a solid, such as frost forming, lower temperatures are what drive the process. Therefore, the correct answer depends entirely on the specific physical or chemical context.
The role of temperature in deposition is not a simple "increase" or "decrease." Instead, temperature acts as an energy control. It can either provide the activation energy needed for chemical reactions to occur, or it can be the energy that must be removed for a gas to become a solid.
The Two Contexts of Deposition
To understand the effect of temperature, we must first distinguish between the two primary meanings of "deposition."
Context 1: Thin-Film Deposition (Engineering)
This process involves creating a solid film on a surface (a substrate) from a vapor. It's a cornerstone of manufacturing in industries like semiconductors, optics, and solar panels. The two main types are Chemical Vapor Deposition (CVD) and Physical Vapor Deposition (PVD).
Context 2: Phase Transition (Physics)
This is the fundamental thermodynamic process where a substance in a gaseous state turns directly into a solid, bypassing the liquid phase. The formation of frost on a cold window is the classic example.
How Temperature Drives Thin-Film Deposition
In manufacturing and research, the goal is often to control the rate and quality of film growth. Temperature is the most critical lever in this process, which typically occurs in three distinct regimes.
The Reaction-Limited Regime
At lower temperatures, the deposition rate is limited by the speed of the chemical reactions on the substrate surface. Increasing the temperature provides more thermal energy, which acts as activation energy. This makes the surface reactions happen much faster, leading to a sharp increase in the deposition rate.
The Mass-Transport-Limited Regime
Once the temperature is high enough that the surface reactions are nearly instantaneous, the bottleneck shifts. The process is now limited by how quickly the reactant gas molecules can travel to the substrate surface. In this regime, the deposition rate plateaus. Further increases in temperature have little to no effect on the rate.
The Desorption-Limited Regime
If the temperature becomes excessively high, the atoms or molecules landing on the surface have too much energy to stick. They begin to re-evaporate, or desorb, back into the gas phase. In this scenario, increasing the temperature further will cause the net deposition rate to decrease significantly.
How Temperature Governs Phase Change Deposition
For the natural phase change from gas to solid, the physics are different. Here, we are not trying to fuel a chemical reaction but rather to force a change in the state of matter.
Removing Energy to Form a Solid
A gas has high internal energy, while a solid has low internal energy. For a gas molecule to become part of a solid structure, it must lose energy. This happens when the gas comes into contact with a surface that is colder than itself, allowing thermal energy to transfer away from the molecule.
The Role of the Dew/Frost Point
This type of deposition only occurs when the surface temperature is at or below the frost point of the gas. Therefore, lower temperatures are required to initiate and sustain the deposition of a solid from a gas.
Understanding the Trade-offs
Simply maximizing the deposition rate by raising the temperature is rarely the best strategy. The choice of temperature involves critical trade-offs that impact the final product.
Rate vs. Quality
Very high deposition rates, often achieved at higher temperatures, can lead to a more disordered and defect-ridden film. Slower, lower-temperature deposition often yields a more uniform, crystalline, and higher-quality film, as atoms have time to settle into their ideal lattice positions.
Substrate and System Limitations
Many substrates, such as plastics or complex electronic devices, cannot withstand high temperatures and would be damaged or destroyed. Furthermore, maintaining high temperatures is energy-intensive and increases operational costs.
Uniformity and Control
Operating in the mass-transport or desorption-limited regimes can be difficult to control. Small temperature variations across the substrate can lead to significant differences in film thickness and quality, which is unacceptable for precision applications like microchips.
Making the Right Choice for Your Goal
Your optimal temperature strategy is defined by your primary objective.
- If your primary focus is high-throughput manufacturing: You will likely operate at the upper end of the reaction-limited regime to maximize deposition rate, carefully balancing speed against the minimum acceptable film quality.
- If your primary focus is a high-quality, defect-free film: You may choose a lower temperature to slow down the growth rate, allowing for a more orderly atomic structure, even at the cost of longer processing times.
- If your primary focus is observing a natural phase change: You must create conditions where a surface is colder than the frost point of the surrounding vapor, as lower temperatures are the direct driver of this process.
Ultimately, mastering deposition requires treating temperature not as a simple switch, but as a precise dial to balance rate, quality, and efficiency.

Summary Table:
| Deposition Context | Primary Temperature Effect | Key Driver |
|---|---|---|
| Thin-Film (CVD/PVD) | Increases rate (to a point) | Provides activation energy for reactions |
| Phase Change (Frost) | Decreases to initiate process | Removes energy for gas-to-solid transition |
Struggling to balance deposition rate with film quality in your lab? KINTEK specializes in precision lab equipment for thin-film deposition processes like CVD and PVD. Our experts can help you select the right furnace or deposition system to precisely control temperature for your specific application—whether you prioritize high-throughput manufacturing or defect-free film quality. Contact our team today to optimize your deposition process and achieve superior results.
Visual Guide
Related Products
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
People Also Ask
- What is the vapor phase deposition technique? A Guide to PVD & CVD Thin-Film Coating Methods
- What is the difference between PECVD and CVD? Unlock the Right Thin-Film Deposition Method
- How does PECVD work? Enable Low-Temperature, High-Quality Thin Film Deposition
- What is the process of vacuum vapor deposition? Mastering CVD and PVD Thin-Film Coating
- What is PECVD in semiconductor? Enable Low-Temperature Thin Film Deposition for ICs



















