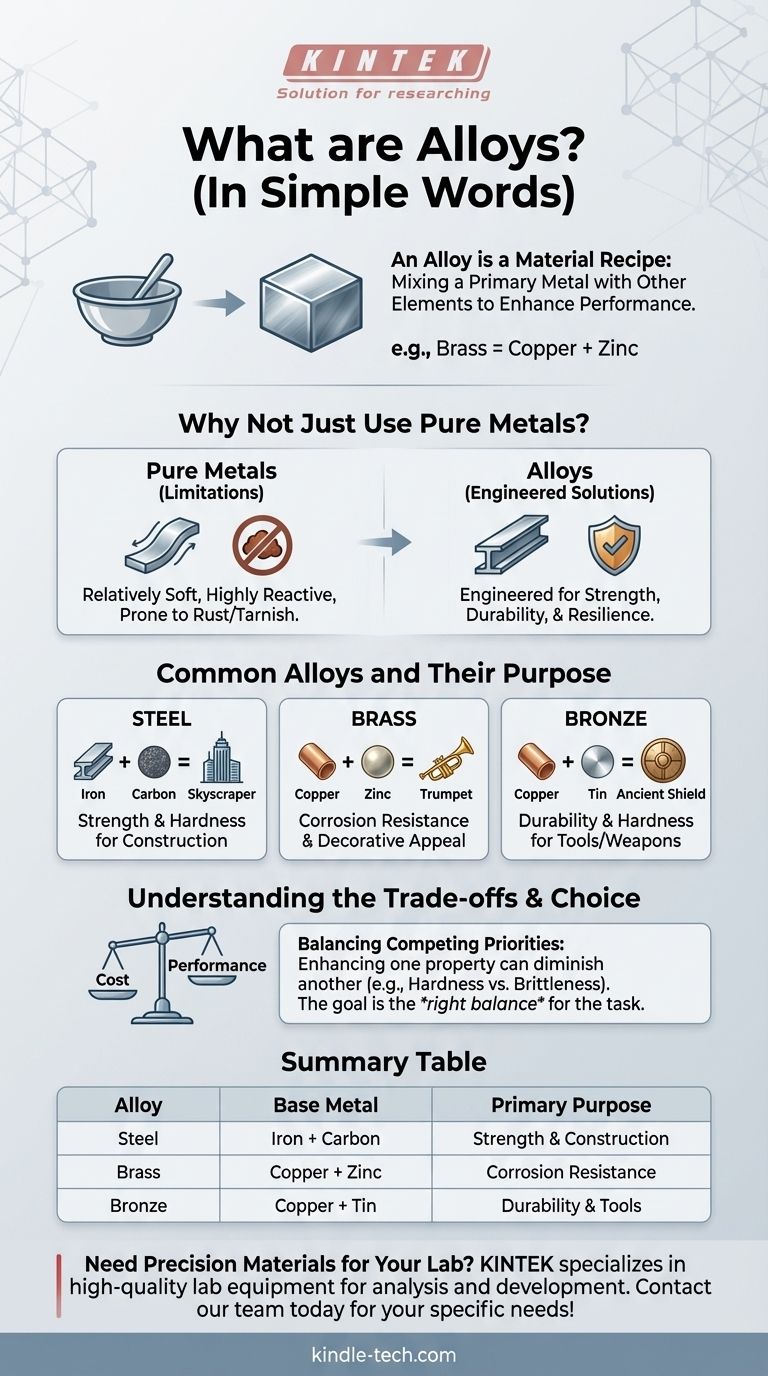
In simple terms, an alloy is a material made by mixing a primary metal with other elements. This process is like creating a recipe to enhance the metal's performance. The added ingredients can be other metals or even non-metallic elements, like carbon. For example, brass is a well-known alloy created by combining two metals: copper and zinc.
The core idea behind an alloy is to engineer a new material with superior properties. We rarely use metals in their pure form because alloys allow us to create materials that are stronger, lighter, or more resistant to rust than the original, base metal could ever be on its own.

Why Not Just Use Pure Metals?
The decision to create and use alloys stems directly from the inherent limitations of pure metals. Alloying is a solution to the natural weaknesses found in raw metallic elements.
The Weakness of Purity
Most pure metals, like iron, aluminum, or copper, are relatively soft in their natural state. They can also be highly reactive with the environment, leading to problems like rust (in the case of iron) or tarnishing.
These properties make them unsuitable for thousands of applications, from constructing skyscrapers to building jet engines, where strength, durability, and resilience are non-negotiable.
Engineering Superior Properties
Alloying is a process of deliberate improvement. By carefully adding other elements, we can fundamentally change the metal's internal structure.
This modification allows us to dial in specific characteristics. We can increase hardness, improve strength, enhance resistance to corrosion, or even change the material's color and melting point.
Common Alloys and Their Purpose
Alloys are all around us, often hidden in plain sight. Each one is designed with a specific job in mind, solving a problem that its base metal could not solve alone.
Steel: The Backbone of Modernity
Steel is perhaps the most important alloy in the world. It is fundamentally an alloy of iron and a small amount of carbon.
That tiny addition of carbon makes iron dramatically stronger and harder, transforming a soft metal into the foundation for everything from bridges and cars to household appliances.
Brass: Decorative and Durable
Brass is an alloy of copper and zinc. The addition of zinc makes the material harder and more resistant to corrosion than pure copper.
This combination also gives it a distinctive bright, gold-like appearance, making it ideal for musical instruments, decorative hardware, and plumbing fixtures that need to resist water damage.
Bronze: A Historical Leap Forward
Bronze, an alloy of copper and tin, was so revolutionary that it has an entire historical period named after it—the Bronze Age.
Bronze is significantly harder and more durable than pure copper. This single innovation allowed for the creation of far superior tools, armor, and weapons, fundamentally changing human civilization.
Understanding the Trade-offs
Creating an alloy is an act of engineering, and like all engineering, it involves balancing competing priorities. There is no single "perfect" material.
Cost vs. Performance
The elements added to create an alloy (known as alloying agents) can be rare or expensive. This can increase the final cost of the material compared to its pure base metal.
Engineers must always weigh the need for higher performance against the constraints of a budget.
Gaining One Thing, Losing Another
Enhancing one property can sometimes diminish another. For example, increasing the hardness of steel can often make it more brittle, meaning it's more likely to crack under a sudden impact.
The goal is not to maximize every property but to create a material with the right balance of properties for a specific task.
Making the Right Choice for Your Goal
Understanding the purpose behind an alloy helps clarify why certain materials are chosen for certain jobs.
- If your primary focus is strength and construction: You will rely on steel, an alloy designed to overcome the softness of pure iron.
- If your primary focus is lightweight performance: You will look to aluminum alloys, which add strength to a naturally light metal for use in aircraft and high-performance vehicles.
- If your primary focus is corrosion resistance: You would choose stainless steel or brass, alloys specifically engineered to withstand exposure to moisture and air without degrading.
Ultimately, an alloy is a testament to our ability to precisely engineer materials to meet the complex demands of our world.
Summary Table:
| Alloy | Base Metal | Key Alloying Element(s) | Primary Purpose |
|---|---|---|---|
| Steel | Iron | Carbon | Strength & Hardness for Construction |
| Brass | Copper | Zinc | Corrosion Resistance & Decorative Appeal |
| Bronze | Copper | Tin | Durability & Hardness for Tools/Weapons |
Need precision-engineered materials for your laboratory applications? The principles of alloying are fundamental to material science. At KINTEK, we specialize in providing the high-quality lab equipment and consumables you need to analyze, test, and develop the next generation of advanced materials. Let our expertise support your research and development goals. Contact our team today to discuss your specific laboratory needs!
Visual Guide
Related Products
- Custom Boron Nitride (BN) Ceramic Parts
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Custom PTFE Teflon Parts Manufacturer for Centrifuge Tubes
- Custom PTFE Teflon Parts Manufacturer for PTFE Ball Valve Seat
- High Temperature Alumina (Al2O3) Furnace Tube for Engineering Advanced Fine Ceramics
People Also Ask
- What are the strengths of brazing? Achieve Strong, Clean, and Precise Metal Joining
- Why are ceramics used for furnace lining? Achieve Peak Efficiency and Durability
- What is the function of a BN inner liner in a graphite mold during Flash Sintering? Master Precise Current Control
- What is a ceramic fiber module? A High-Temperature Insulation System for Rapid Furnace Lining
- What kind of insulation is used in a furnace? A Guide to Optimizing Thermal Efficiency and Performance










