At its core, a heat-resistant crucible is a specialized container engineered to withstand extreme temperatures without melting, breaking, or reacting with the substance it holds. They are essential tools in metallurgy, material science, and chemistry for melting metals and other materials. Crucibles are typically made from ceramic or composite materials like clay-graphite and silicon carbide, which have melting points far higher than the materials being processed.
A crucible is not merely a heat-proof bowl; it is a critical piece of equipment whose material properties—from thermal shock resistance to chemical inertness—directly determine the success and purity of any high-temperature process.

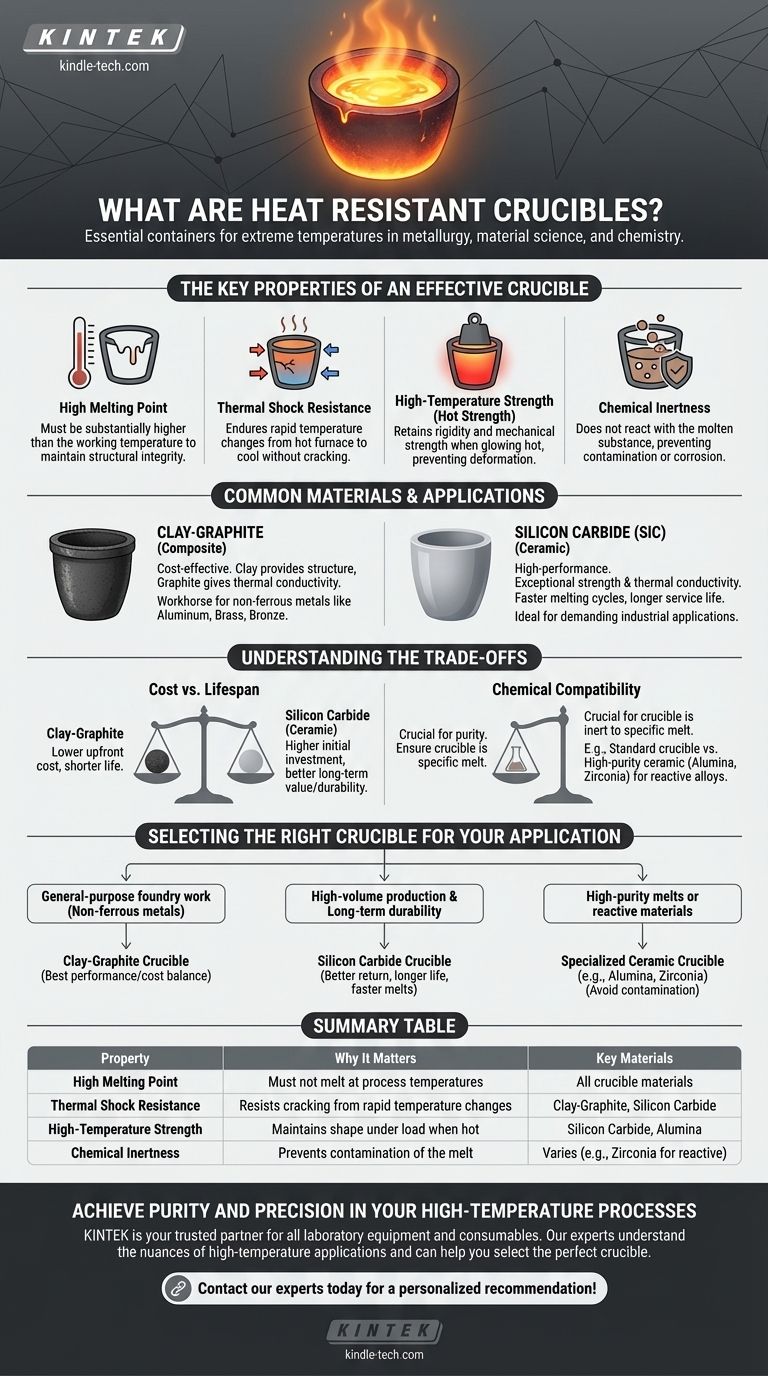
The Key Properties of an Effective Crucible
To perform reliably under extreme conditions, a crucible must possess a specific set of characteristics. The material's melting point is just the beginning of the story.
High Melting Point
The most fundamental requirement is that the crucible's melting point is substantially higher than the working temperature of the material inside it. This ensures the container maintains its solid form and structural integrity.
Thermal Shock Resistance
Materials can crack or shatter when subjected to rapid temperature changes. A crucible must have excellent thermal shock resistance to endure being placed into a hot furnace and later removed to cool without failing.
High-Temperature Strength (Hot Strength)
Many materials soften and lose their strength long before they reach their melting point. A quality crucible retains its rigidity and mechanical strength even when glowing hot, preventing it from deforming under the weight of the molten contents.
Chemical Inertness
It is critical that the crucible material does not chemically react with the molten substance it contains. A reaction can contaminate the final product or, in aggressive cases, corrode and destroy the crucible itself.
Common Materials and Their Applications
The choice of crucible material is dictated by the temperature required, the substance being melted, and the budget.
Clay-Graphite
This is a common and cost-effective composite material. The clay provides the structural form, while the embedded graphite flakes provide excellent thermal conductivity and resistance to thermal shock. They are a workhorse for melting non-ferrous metals like aluminum, brass, and bronze.
Silicon Carbide (SiC)
Silicon carbide is a higher-performance ceramic known for its exceptional strength at high temperatures and superior thermal conductivity. This allows for faster melting cycles and gives SiC crucibles a longer service life, making them ideal for demanding industrial applications.
Understanding the Trade-offs
Selecting a crucible involves balancing performance requirements with practical limitations. No single type is perfect for every task.
Cost vs. Lifespan
Clay-graphite crucibles are less expensive upfront but may have a shorter operational life than more advanced options. Silicon carbide crucibles represent a higher initial investment but often provide better long-term value due to their durability.
Chemical Compatibility
The primary trade-off is ensuring the crucible is inert to the specific material you are melting. For example, while excellent for many metals, a standard crucible might not be suitable for melting highly reactive alloys or specialized glass, which may require a high-purity ceramic like alumina or zirconia.
Selecting the Right Crucible for Your Application
Your choice should be directly informed by the demands of your specific process.
- If your primary focus is general-purpose foundry work with non-ferrous metals: A clay-graphite crucible offers the best balance of performance and cost.
- If your primary focus is high-volume production and long-term durability: Investing in a silicon carbide crucible will likely yield a better return through longer service life and faster melt times.
- If your primary focus is high-purity melts or reactive materials: You must select a specialized ceramic crucible (like alumina or zirconia) specifically rated for your application to avoid contamination.
Ultimately, choosing the correct heat-resistant crucible is a foundational step for achieving clean, successful, and repeatable results in any high-temperature work.
Summary Table:
| Property | Why It Matters | Key Materials |
|---|---|---|
| High Melting Point | Must not melt at process temperatures | All crucible materials |
| Thermal Shock Resistance | Resists cracking from rapid temperature changes | Clay-Graphite, Silicon Carbide |
| High-Temperature Strength | Maintains shape under load when hot | Silicon Carbide, Alumina |
| Chemical Inertness | Prevents contamination of the melt | Varies by material (e.g., Zirconia for reactive metals) |
Achieve Purity and Precision in Your High-Temperature Processes
Selecting the right crucible is critical for the success of your melting, calcination, or synthesis work. The wrong choice can lead to contamination, crucible failure, and inconsistent results.
KINTEK is your trusted partner for all laboratory equipment and consumables. Our experts understand the nuances of high-temperature applications and can help you select the perfect crucible—whether you need cost-effective clay-graphite for foundry work, durable silicon carbide for industrial production, or high-purity ceramics for sensitive materials.
We provide more than just products; we provide solutions that ensure the integrity of your materials and the efficiency of your lab.
Let's discuss your specific application needs. Contact our experts today for a personalized recommendation!
Visual Guide
Related Products
- Custom Machined and Molded PTFE Teflon Parts Manufacturer with PTFE Crucible and Lid
- Engineering Advanced Fine Ceramics Alumina Al2O3 Crucible With Lid Cylindrical Laboratory Crucible
- Engineering Advanced Fine Ceramics Alumina Crucibles (Al2O3) for Thermal Analysis TGA DTA
- Alumina Al2O3 Ceramic Crucible Semicircle Boat with Lid for Engineering Advanced Fine Ceramics
- Arc-Shaped Alumina Ceramic Crucible High Temperature Resistant for Engineering Advanced Fine Ceramics
People Also Ask
- Why are alumina crucibles typically selected for the TG/DTG of sodium-functionalized biochar? Ensure Accurate Analysis
- Why are Platinum/Gold (Pt/Au) crucibles selected for silver phosphate glass? Ensure Maximum Purity in Glass Synthesis
- What are crucibles for laboratory use? Essential Guide to High-Temperature Containment
- What are the advantages of using high-purity alumina crucibles for YSC powders? Ensure Chemical Purity & Stability
- What is the difference between a crucible and a furnace? Understanding the Heat Source and Container Partnership
- What is an industrial crucible? A Guide to High-Temperature Melting Vessels
- Why Use Alumina Crucibles in Quartz Tubes for 1273 K Annealing? Protect Your Alloy Purity & Prevent Oxidation
- What is the role of grinding spent catalysts in a ceramic crucible? Unlock 99% Leaching Recovery Rates



















