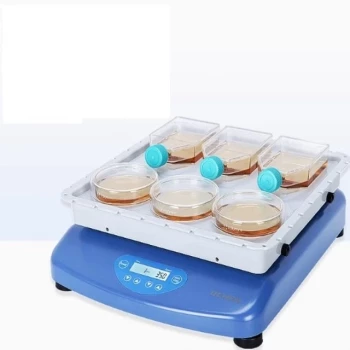
In fast pyrolysis, the most common catalysts are commercial silicon and zeolite-based materials. However, their direct application to biomass conversion is challenging because the large, natural polymer molecules found in biomass are often too bulky to effectively interact with the catalyst's small internal structures.
The central challenge in catalytic fast pyrolysis is a physical mismatch: standard catalysts have micropores designed for small petrochemical molecules, while biomass yields large, complex molecules that block these pores. The solution lies in engineering catalysts with a hierarchical, multi-level pore structure to accommodate this difference in scale.

Why Standard Catalysts Fall Short for Biomass
The catalysts that excel in traditional petrochemical refining, like zeolites, were not designed for the unique challenges posed by biomass. This fundamental mismatch is the primary hurdle in producing high-quality biofuels and chemicals through catalytic pyrolysis.
The Biomass Size Problem
Biomass is composed of large natural polymers like cellulose, hemicellulose, and lignin. When these materials are rapidly heated during pyrolysis, they break down into a wide range of bulky, oxygenated organic molecules. These molecules are significantly larger and more complex than the hydrocarbons found in crude oil.
The Pore Structure Limitation
Zeolites and other conventional catalysts are prized for their microporous structure, which contains active sites where chemical reactions occur. While highly effective for small molecules that can easily diffuse inside, these narrow pores are a major bottleneck for the larger molecules derived from biomass, leading to blockages and reduced efficiency.
The Solution: Engineering a Better Catalyst
To overcome the limitations of conventional catalysts, the focus has shifted to creating advanced materials specifically designed for bulky biomass derivatives. The key is to control the flow of molecules at multiple scales.
Introducing Multidimensional Porosity
The most effective modern catalysts couple the traditional micropores with a secondary network of larger pores. This creates a hierarchical or multidimensional structure with micro, meso, and macro pores, each serving a distinct purpose.
How Hierarchical Structures Work
Think of this structure as a city's road system. The large macropores act as superhighways, granting bulky biomass molecules initial access deep into the catalyst particle. The intermediate mesopores serve as city streets, distributing these molecules further.
Finally, the molecules reach the small micropores, which are like driveways leading to the catalytic "active sites" where the desired chemical conversions happen. This prevents traffic jams at the surface and ensures the entire catalyst volume is utilized.
Understanding the Inherent Challenges
While hierarchical catalysts offer a clear solution, their design and implementation come with practical considerations. Understanding these trade-offs is crucial for developing commercially viable processes.
Catalyst Deactivation and Coking
The high temperatures and complex molecules involved in pyrolysis can lead to the formation of carbonaceous deposits, or "coke," on the catalyst surface. This deactivates the catalyst by physically blocking the pores and covering active sites. While hierarchical pores can delay this process, it remains a significant operational challenge.
Complexity and Cost
Creating sophisticated, multi-level pore structures is a more complex and expensive process than producing standard commercial zeolites. The long-term performance benefits, such as higher yields of valuable products and longer catalyst lifespan, must outweigh this initial investment.
Making the Right Choice for Your Goal
The optimal catalytic strategy depends entirely on the nature of the feedstock and the desired final product.
- If your primary focus is converting raw, bulky biomass directly: A hierarchical catalyst with a well-defined network of micro, meso, and macropores is essential to manage the molecular traffic and prevent rapid deactivation.
- If your primary focus is upgrading smaller, pre-treated bio-oil vapors: A conventional microporous catalyst, such as a standard zeolite, may be perfectly suitable and more cost-effective.
Ultimately, effective catalytic pyrolysis demands that the catalyst's architecture is intelligently matched to the scale of the molecules it is designed to transform.
Summary Table:
| Catalyst Type | Key Feature | Primary Use Case | Main Challenge |
|---|---|---|---|
| Standard Zeolites | Microporous structure | Petrochemical refining, upgrading pre-treated bio-oil | Pore blockage by large biomass molecules |
| Hierarchical Catalysts | Multi-level pores (micro, meso, macro) | Direct conversion of raw, bulky biomass | Higher complexity and cost |
Optimize your biomass conversion process with the right catalysts. The challenge of converting bulky biomass into valuable biofuels requires specialized equipment and expertise. KINTEK specializes in lab equipment and consumables, providing the tools and support needed to develop and scale your catalytic fast pyrolysis research. Our solutions help you achieve higher yields and longer catalyst lifespans. Contact us today to discuss how we can enhance your laboratory's efficiency and success. Get in touch with our experts
Visual Guide
Related Products
- Custom PTFE Teflon Parts Manufacturer for Three-Necked Round Bottom Flask
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Platinum Auxiliary Electrode for Laboratory Use
- Proton Exchange Membrane for Batteries Lab Applications
- Customizable PEM Electrolysis Cells for Diverse Research Applications
People Also Ask
- Why is slender PTFE tubing necessary for flow control in multi-channel catalyst aging? Ensure Equal Gas Distribution
- What maintenance procedures are recommended for a PTFE cleaning basket? Extend Equipment Life & Ensure Process Purity
- Is PTFE corrosion resistant? Discover the Ultimate Chemical Resistance for Your Lab
- What are the primary reasons for selecting PTFE as a matrix? Enhance Composites with Carbon Nanotube Reinforcement
- What are the advantages of using PTFE lined tubing? Optimize Sample Integrity & Reduce Memory Effects