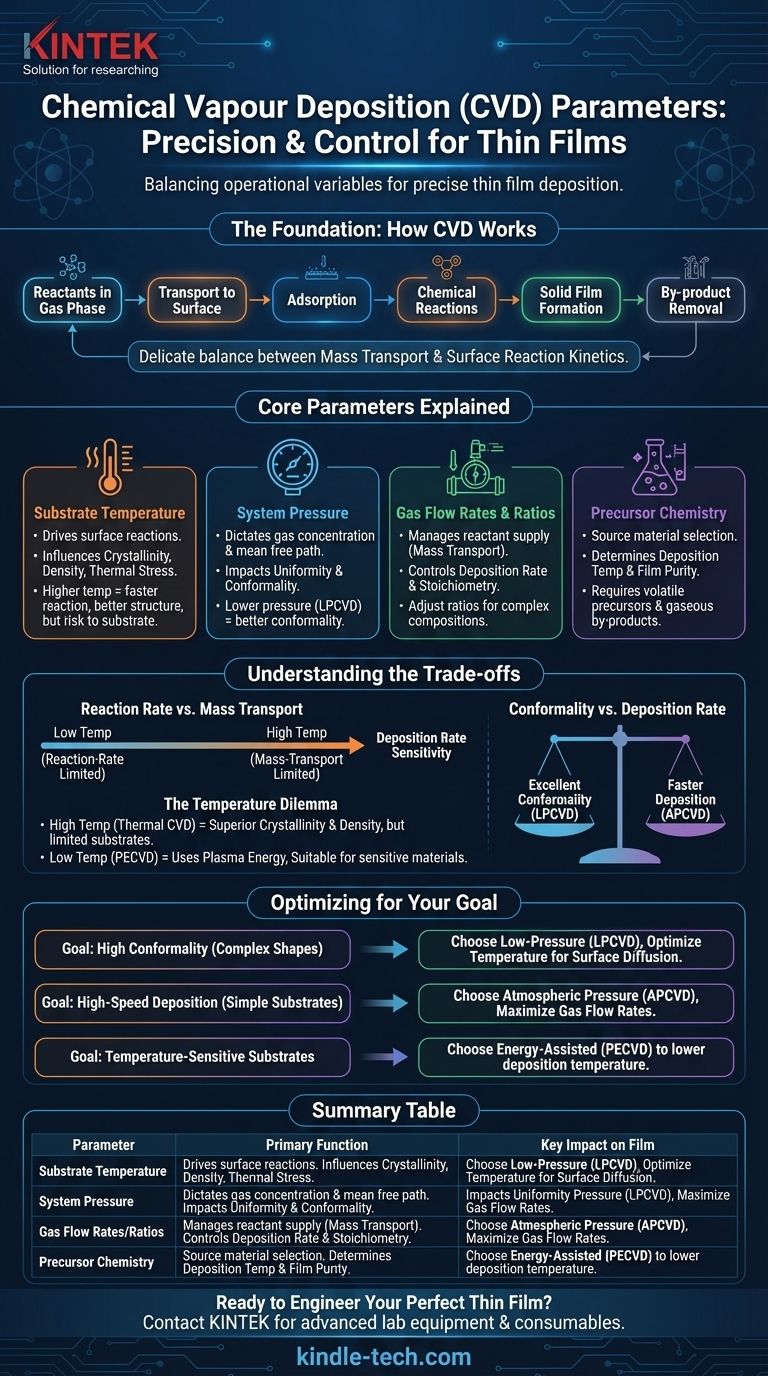
The core parameters of Chemical Vapor Deposition (CVD) are the operational variables you adjust to control the deposition of a thin film. The most critical of these are substrate temperature, system pressure, the flow rates of reactant gases, and the specific chemical precursors used. Mastering these parameters allows for precise control over the final film's chemical composition, crystal structure, and physical properties.
The challenge of CVD is not merely knowing what the parameters are, but understanding that they are interdependent. The process is a delicate balance between mass transport (delivering reactants to the surface) and surface reaction kinetics (the chemical reactions forming the film), with each parameter influencing this balance.

The Foundation: How CVD Works
Before manipulating the parameters, it's essential to understand the fundamental process they control. CVD is not a single event but a sequence of steps that must occur correctly to form a high-quality film.
The Deposition Sequence
The entire process involves reactants in a gas phase reacting to form a solid film on a substrate. This sequence includes the transport of gases to the surface, their adsorption, chemical reactions, and the removal of by-products. Each parameter directly influences one or more of these stages.
The Primary Control Parameters Explained
The properties of your final film—from its purity and density to its crystalline structure—are a direct result of how you set and balance the following primary parameters.
Substrate Temperature
Temperature provides the thermal energy required to drive the chemical reactions on the substrate surface. It is often the most critical parameter for controlling the film's structure.
Higher temperatures generally increase the reaction rate, improve film density, and can lead to better crystallinity. However, excessively high temperatures can damage temperature-sensitive substrates or introduce unwanted thermal stress.
System Pressure
The pressure inside the reaction chamber dictates the concentration and mean free path of the gas molecules. This has a profound impact on film uniformity and its ability to coat complex shapes.
Different pressure regimes define the type of CVD, such as Atmospheric Pressure CVD (APCVD) or Low-Pressure CVD (LPCVD). Lower pressures increase the mean free path of gas molecules, often resulting in more uniform and conformal coatings.
Gas Flow Rates and Ratios
The rate at which precursor gases are introduced into the chamber controls the supply of reactants. This is the primary lever for managing the mass transport side of the CVD balance.
The ratio of different gases is also critical, as it determines the stoichiometry (the proportion of elements) of the final film. Adjusting these ratios allows for the deposition of complex alloys and compounds with specific chemical compositions.
Precursor Chemistry
The choice of the chemical source material, or precursor, is a foundational parameter set before the process even begins. The precursor must be volatile enough to be transported as a gas and must decompose cleanly at the desired deposition temperature.
The by-products of the precursor reaction must also be gaseous so they can be easily removed from the chamber without contaminating the growing film.
Understanding the Trade-offs
Optimizing a CVD process is rarely straightforward. Adjusting one parameter to improve a specific film characteristic often has a negative effect on another. Understanding these trade-offs is key to successful deposition.
Reaction Rate vs. Mass Transport
At lower temperatures, the deposition rate is typically limited by the speed of the chemical reactions on the surface (reaction-rate limited). In this regime, the process is highly sensitive to temperature changes.
At higher temperatures, the reactions happen so quickly that the process becomes limited by how fast the reactant gases can be delivered to the surface (mass-transport limited). Here, the deposition rate is more sensitive to gas flow rates and pressure.
The Temperature Dilemma
While high temperatures can produce films with superior crystallinity and density, they are a major limitation. Many substrates cannot withstand the typical 850-1100°C temperatures of thermal CVD. This has led to the development of methods like Plasma-Enhanced CVD (PECVD), which use plasma to provide the reaction energy, allowing for deposition at much lower temperatures.
Conformality vs. Deposition Rate
Achieving excellent conformality, or the ability to uniformly coat complex, non-flat surfaces, is a major strength of CVD. This is often best achieved at low pressures (LPCVD), where gas molecules can diffuse more freely into intricate features.
However, this often comes at the cost of a slower deposition rate compared to high-pressure or atmospheric systems.
Optimizing Parameters for Your Goal
Your ideal parameter set depends entirely on the desired outcome for your film. The key is to align your process variables with your primary objective.
- If your primary focus is high-quality, uniform films on complex shapes: You should lean towards a low-pressure (LPCVD) process, carefully optimizing temperature to balance reaction rate with surface diffusion for maximum conformality.
- If your primary focus is high-speed deposition on simple substrates: An atmospheric pressure (APCVD) system may be more efficient, with a focus on maximizing gas flow rates to operate in the mass-transport limited regime.
- If you are working with temperature-sensitive substrates like polymers or certain electronics: You must use an energy-assisted process like PECVD to lower the deposition temperature while still providing enough energy for the chemical reaction.
By systematically controlling these core parameters, you can move from simply depositing a material to precisely engineering a thin film with tailored properties.
Summary Table:
| Parameter | Primary Function | Key Impact on Film |
|---|---|---|
| Substrate Temperature | Drives surface reaction kinetics | Controls crystallinity, density, and stress |
| System Pressure | Dictates gas concentration & mean free path | Influences uniformity and conformality |
| Gas Flow Rates/Ratios | Manages reactant supply (mass transport) | Determines deposition rate and stoichiometry |
| Precursor Chemistry | Defines the source material for the film | Sets fundamental deposition temperature and purity |
Ready to Engineer Your Perfect Thin Film?
Mastering the delicate balance of CVD parameters is the key to achieving your specific film goals, whether it's high conformality on complex shapes, high-speed deposition, or low-temperature processing for sensitive substrates. KINTEK specializes in providing the advanced lab equipment and consumables you need to precisely control every aspect of your CVD process.
Our experts can help you select the right system and optimize your parameters for superior results. Contact our team today to discuss your application and discover how KINTEK can advance your laboratory's capabilities.
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
People Also Ask
- What is the difference between plasma CVD and thermal CVD? Choose the Right Method for Your Substrate
- How are thin films deposited? A Guide to PVD vs. CVD Methods for Your Application
- Why is a Matching Network Indispensable in RF-PECVD for Siloxane Films? Ensure Stable Plasma and Uniform Deposition
- Can plasma enhanced CVD deposit metals? Why PECVD is rarely used for metal deposition
- How do PECVD systems improve DLC coatings on implants? Superior Durability and Biocompatibility Explained



















