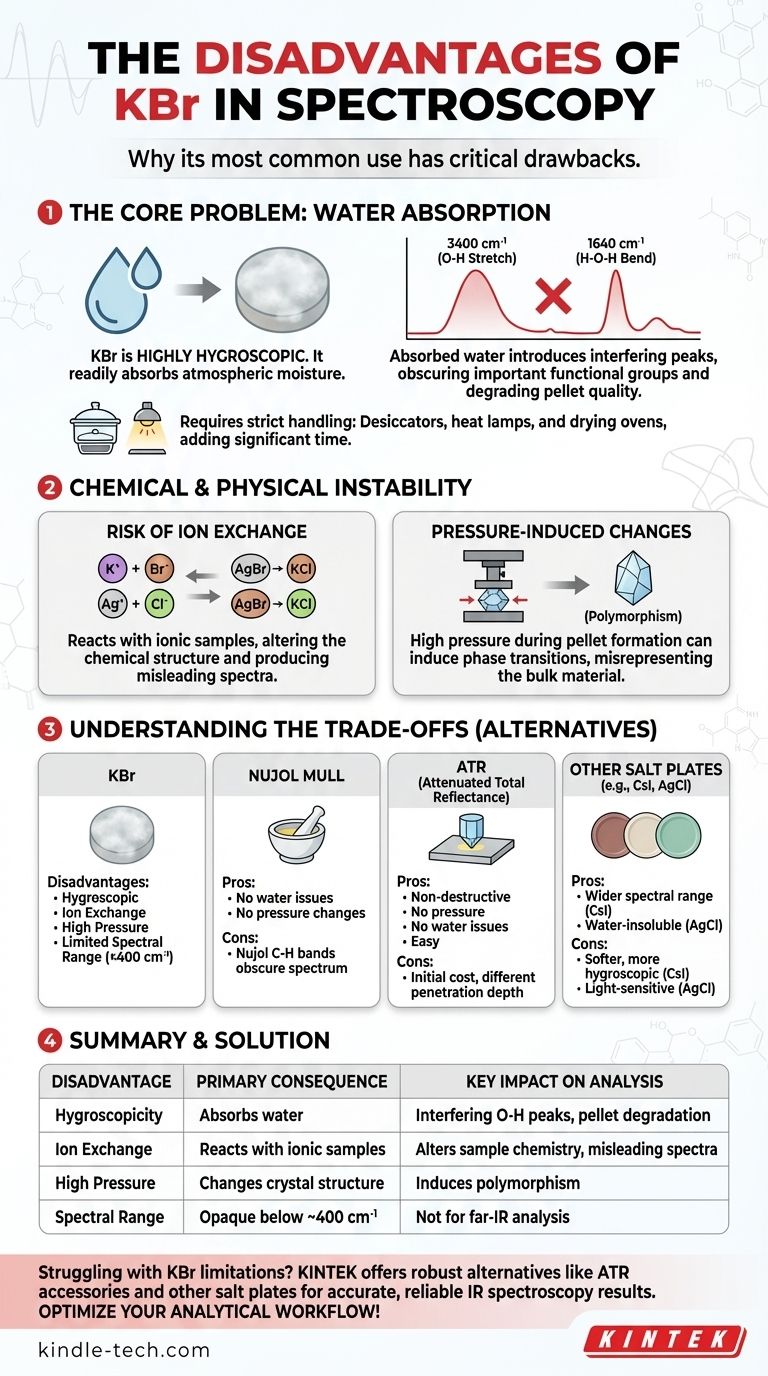
While widely used in spectroscopy, the primary disadvantage of Potassium Bromide (KBr) is that it is highly hygroscopic, meaning it readily absorbs moisture from the atmosphere. This absorbed water introduces significant interfering peaks into an infrared spectrum and can physically degrade the sample pellet over time, compromising the quality and accuracy of your results.
KBr's tendency to absorb atmospheric water is its most common practical drawback. However, its potential for unwanted chemical reactions and its limited spectral range are equally critical limitations that can invalidate analytical results if not properly understood.

The Core Problem: Water Absorption
The most frequent and frustrating issue when working with KBr is its affinity for water. This single property creates several distinct problems during sample preparation and analysis for IR spectroscopy.
How Water Interferes with Your Spectrum
Absorbed water is not spectrally silent. It produces two very distinct and prominent absorption bands in a typical mid-IR spectrum.
You will see a very broad, strong band around 3400 cm⁻¹ due to the O-H stretching vibration and a sharper, medium-intensity band around 1640 cm⁻¹ from the H-O-H bending vibration.
These water peaks can easily obscure or overlap with important functional group signals from your actual sample, such as N-H and O-H stretches, as well as some C=O and C=C vibrations.
The Impact on Pellet Quality
Moisture doesn't just affect the spectrum; it affects the physical integrity of the KBr pellet. As the salt absorbs water, a pressed disc will slowly become cloudy and opaque.
This cloudiness scatters the IR beam, which reduces the amount of light reaching the detector. The result is a noisy spectrum with poor signal quality, making subtle peaks impossible to identify. Over time, the pellet may even crumble.
Necessary Handling Procedures
To combat moisture, KBr requires strict handling protocols. The powder must be stored in a desiccator and should be ground to a powder under a heat lamp or in a dry box to minimize water exposure.
Before use, the KBr powder must be dried in an oven for several hours. This adds significant time and effort to the sample preparation process.
Chemical and Physical Instability
Beyond water, the chemical and physical nature of KBr can introduce other, more subtle errors into your analysis. These issues are often harder to detect and can lead to incorrect interpretations of your data.
The Risk of Ion Exchange
KBr is an ionic salt (K⁺ Br⁻). If your sample is also an ionic compound, particularly a halide salt, an ion-exchange reaction can occur within the pellet.
For example, mixing a sample of silver chloride (AgCl) with KBr can result in the formation of AgBr and KCl. Your resulting spectrum would be of this new mixture, not your original sample. This is a critical issue for many inorganic and organometallic compounds.
Pressure-Induced Sample Changes
Creating a KBr pellet requires applying several tons of pressure. This intense pressure can sometimes induce a phase transition in crystalline samples, a phenomenon known as polymorphism.
You may inadvertently change the crystal structure of your analyte during sample preparation. The resulting spectrum would be of this new polymorph, which may not be representative of your bulk material.
Understanding the Trade-offs
KBr's disadvantages become clearer when compared to alternative sample preparation methods for IR spectroscopy.
KBr vs. Nujol Mull
A Nujol mull involves grinding the sample with a mineral oil (Nujol). This method avoids the issues of water absorption and pressure-induced changes. However, the Nujol itself has C-H absorption bands that will obscure parts of the spectrum.
KBr vs. Attenuated Total Reflectance (ATR)
ATR is a modern technique that analyzes the surface of a sample directly without any preparation. It is non-destructive, requires no pressure, and is unaffected by atmospheric moisture. The main trade-off is the initial cost of the ATR accessory, and spectra can sometimes differ slightly from transmission spectra due to varying penetration depth.
KBr vs. Other Salt Plates (CsI, AgCl)
For analysis in the far-IR region (below 400 cm⁻¹), KBr is not transparent and therefore unusable. Cesium Iodide (CsI) offers a wider spectral range but is even softer and more hygroscopic than KBr. Silver Chloride (AgCl) is water-insoluble and useful for reactive samples but is soft, photosensitive, and more expensive.
Making the Right Choice for Your Goal
Selecting the correct sample preparation method is essential for acquiring accurate data. Your choice should be dictated by the nature of your sample and the goals of your analysis.
- If your primary focus is routine mid-IR analysis of stable, non-ionic organic compounds: KBr is a cost-effective choice, provided you diligently follow procedures to control for moisture.
- If you are analyzing ionic salts or coordination compounds: You must consider alternatives like a Nujol mull or an AgCl pellet to prevent ion-exchange reactions.
- If your sample is sensitive to pressure or you suspect polymorphism: ATR is a far superior, non-destructive alternative that eliminates this risk entirely.
- If you need to analyze in the far-IR region (below 400 cm⁻¹): KBr is unusable; you must use a material with the appropriate transparency, such as CsI or polyethylene.
Understanding these limitations allows you to select the appropriate technique, ensuring the integrity and accuracy of your spectroscopic data.
Summary Table:
| Disadvantage | Primary Consequence | Key Impact on Analysis |
|---|---|---|
| Hygroscopicity | Absorbs atmospheric water | Introduces interfering O-H peaks; degrades pellet clarity |
| Ion Exchange | Reacts with ionic samples | Alters sample chemistry; produces misleading spectra |
| High Pressure | Can change crystal structure | Induces polymorphism; misrepresents bulk material |
| Spectral Range | Opaque below ~400 cm⁻¹ | Not suitable for far-IR analysis |
Struggling with KBr limitations in your lab? KINTEK specializes in laboratory equipment and consumables, offering robust alternatives like ATR accessories and alternative salt plates to ensure accurate, reliable IR spectroscopy results. Let our experts help you select the ideal sample preparation method for your specific application. Contact us today to optimize your analytical workflow!
Visual Guide
Related Products
- kbr pellet press 2t
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- Automatic Laboratory Hydraulic Press for XRF & KBR Pellet Press
- Laboratory Hydraulic Press Lab Pellet Press Machine for Glove Box
- Laboratory Manual Hydraulic Pellet Press for Lab Use
People Also Ask
- What are the functions of a laboratory hydraulic press in cathode research? Optimize High-Nickel Electrode Preparation
- What are the advantages and disadvantages of presses? A Guide to Lab Filter Presses for R&D
- What is the use of KBr pellets? Achieve Clear FTIR Analysis of Solid Samples
- Why are KBr pellets used in FTIR? Achieve Clear, Accurate Solid Sample Analysis
- What function does a laboratory hydraulic press serve in the preparation of LLZTO ceramic electrolyte pellets?
- What mechanism would cause hydraulic failure? Prevent System Breakdown with Proactive Fluid Care
- What is the purpose of specialized molding and pressure systems? Ensure Refractory Structural Homogeneity
- What happens if hydraulic pressure is too low? Avoid System Failure and Costly Damage



















