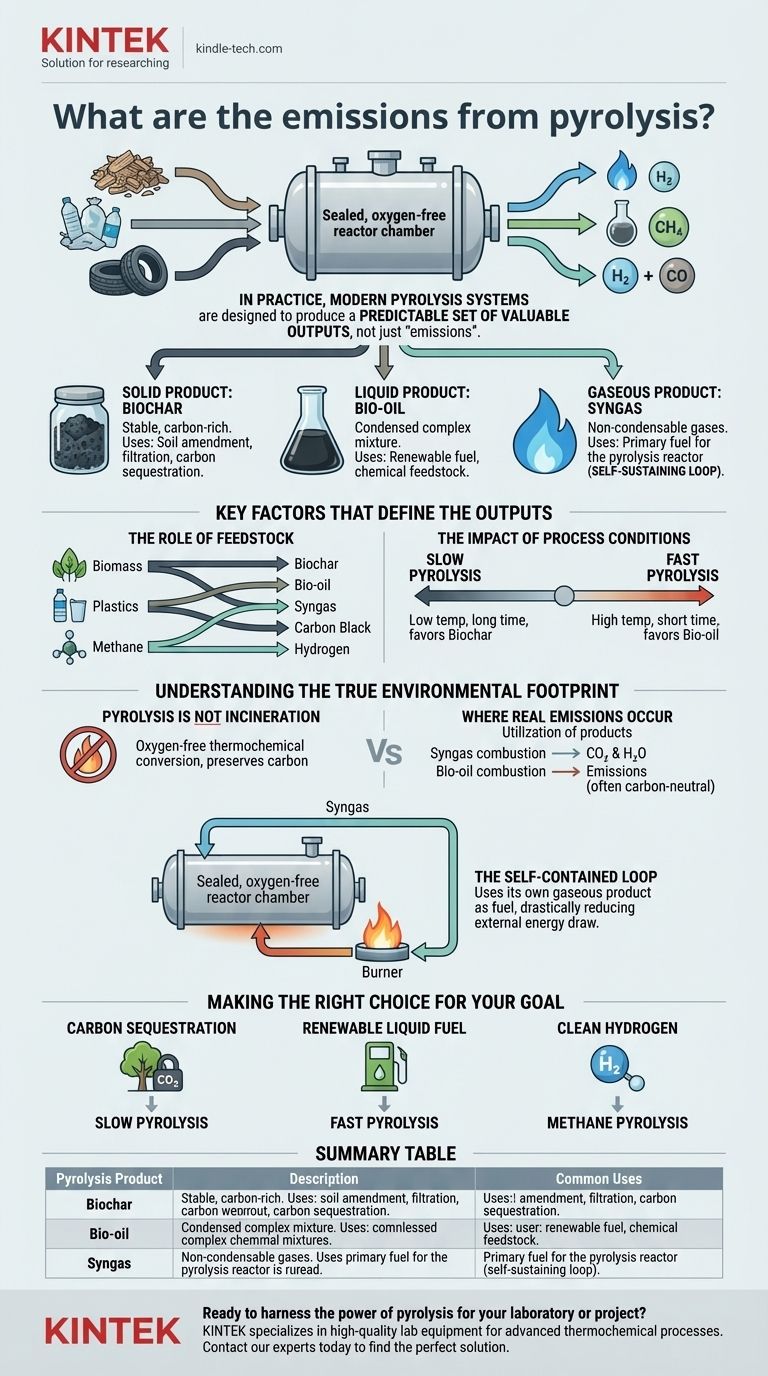
In practice, modern pyrolysis systems are designed not to have "emissions" in the traditional sense of pollution, but rather to produce a predictable set of valuable outputs. The process thermally decomposes material in an oxygen-free environment, yielding three distinct product streams: a solid (biochar), a liquid (bio-oil), and a gas (syngas). The precise composition of these products is highly dependent on the input material and process conditions.
The core misunderstanding is to view pyrolysis outputs as "emissions." It is more accurate to see them as a slate of controllable products. The true environmental footprint is not determined by the pyrolysis process itself, but by how these resulting solids, liquids, and gases are subsequently used or managed.
Deconstructing the Outputs: The Three Core Products
Pyrolysis does not burn material; it deconstructs it. Because this occurs in a sealed, oxygen-starved chamber, the typical byproducts of combustion (like ash, soot, dioxins, or NOx) are not formed. Instead, the input material is transformed.
The Solid Product: Biochar
The primary solid output is a stable, carbon-rich material known as biochar (from biomass) or coke (from other materials like tires).
This is not ash. It is the original carbon structure of the feedstock, with most of the volatile compounds removed. Its uses are extensive, including soil amendment, water filtration, and creating carbon-based materials.
The Liquid Product: Bio-oil
As the process gases cool, a dense, dark liquid known as pyrolysis oil (or bio-oil) condenses. This is a complex mixture of water, tars, and hundreds of different organic compounds.
Bio-oil can be a valuable product. It can be combusted to generate heat and power, or it can be upgraded and refined into advanced transportation biofuels and specialty chemicals.
The Gaseous Product: Syngas
The non-condensable gases that remain after the bio-oil is separated form a mixture called synthesis gas, or syngas.
This gas is typically composed of hydrogen (H₂), methane (CH₄), carbon monoxide (CO), and carbon dioxide (CO₂). In most modern pyrolysis plants, this syngas is not released. Instead, it is looped back and used as the primary fuel to generate heat for the pyrolysis reactor, making the process largely self-sustaining.
Key Factors That Define the Outputs
The ratio and composition of these three products are not fixed. They can be deliberately manipulated by tuning the process, making pyrolysis a uniquely flexible conversion technology.
The Role of Feedstock
The input material, or feedstock, is the single most important factor.
- Biomass pyrolysis yields biochar, bio-oil, and syngas.
- Plastics pyrolysis yields a different profile of oil, gas, and a solid carbon residue.
- Methane pyrolysis is a specialized process designed to produce two clean outputs: solid "carbon black" and valuable **hydrogen gas (H₂) **, with no direct CO₂ emissions.
The Impact of Process Conditions
Engineers can "steer" the process to favor one output over another by controlling temperature and heating rate.
- Slow Pyrolysis: Lower temperatures and longer processing times maximize the yield of biochar. This is ideal for carbon sequestration goals.
- Fast Pyrolysis: High temperatures and very short processing times maximize the yield of bio-oil. This is the preferred method for producing liquid biofuels.
Understanding the True Environmental Footprint
The critical distinction to make is between the pyrolysis process and the subsequent use of its products. This is where emissions are truly generated and must be managed.
Pyrolysis is Not Incineration
Incineration is burning with excess oxygen, which breaks down materials and releases energy, but also produces CO₂ and potential pollutants. Pyrolysis is a thermochemical conversion without oxygen, which preserves chemical complexity and carbon in the solid and liquid products.
Where Real Emissions Occur
The primary emissions concern is linked to the utilization of the products.
- When syngas is burned to heat the reactor, its combustion releases emissions (mostly CO₂ and water), which must be managed like any other fuel combustion process.
- When bio-oil is burned for energy, it also produces combustion emissions. The benefit is that it is often considered a carbon-neutral fuel, as the carbon originally came from atmospheric CO₂ via photosynthesis.
The Self-Contained Loop
The most significant feature of an industrial pyrolysis system is its ability to use its own gaseous product as fuel. This internal loop means the primary external energy requirement is only for starting up the system. It contains the most volatile outputs and uses them productively, drastically reducing the facility's external energy draw and overall emissions profile.
Making the Right Choice for Your Goal
Pyrolysis is not one-size-fits-all; it is a platform technology that can be optimized for specific outcomes.
- If your primary focus is carbon sequestration: You will use slow pyrolysis to convert biomass into stable biochar, locking carbon in a solid form for centuries.
- If your primary focus is producing renewable liquid fuel: You will use fast pyrolysis to maximize bio-oil yield, which can then be refined for use as a heating oil or advanced biofuel.
- If your primary focus is creating clean hydrogen: You will use methane pyrolysis to split natural gas into valuable hydrogen gas and solid carbon, avoiding the direct CO₂ emissions of traditional steam-methane reforming.
Ultimately, pyrolysis empowers us to reframe "waste" and "emissions" by converting low-value materials into high-value, controllable products.

Summary Table:
| Pyrolysis Product | Description | Common Uses |
|---|---|---|
| Biochar (Solid) | Stable, carbon-rich solid from biomass. | Soil amendment, filtration, carbon sequestration. |
| Bio-oil (Liquid) | Condensed liquid from process gases. | Renewable fuel, chemical feedstock. |
| Syngas (Gas) | Non-condensable gas mixture (H₂, CH₄, CO). | Fuel for the pyrolysis reactor (self-sustaining loop). |
Ready to harness the power of pyrolysis for your laboratory or project? KINTEK specializes in high-quality lab equipment and consumables for advanced thermochemical processes. Whether you are researching biochar for carbon sequestration, optimizing bio-oil yields, or developing clean hydrogen production, our reliable tools are designed to support your innovation. Contact our experts today to find the perfect solution for your specific pyrolysis application and transform your material challenges into valuable opportunities.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- 1700℃ Muffle Oven Furnace for Laboratory
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
People Also Ask
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs
- What are the different types of pyrolysis machines? Choose the Right System for Your Output
- What are the components of biomass pyrolysis? A Complete Guide to the System, Products, and Process
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success



















