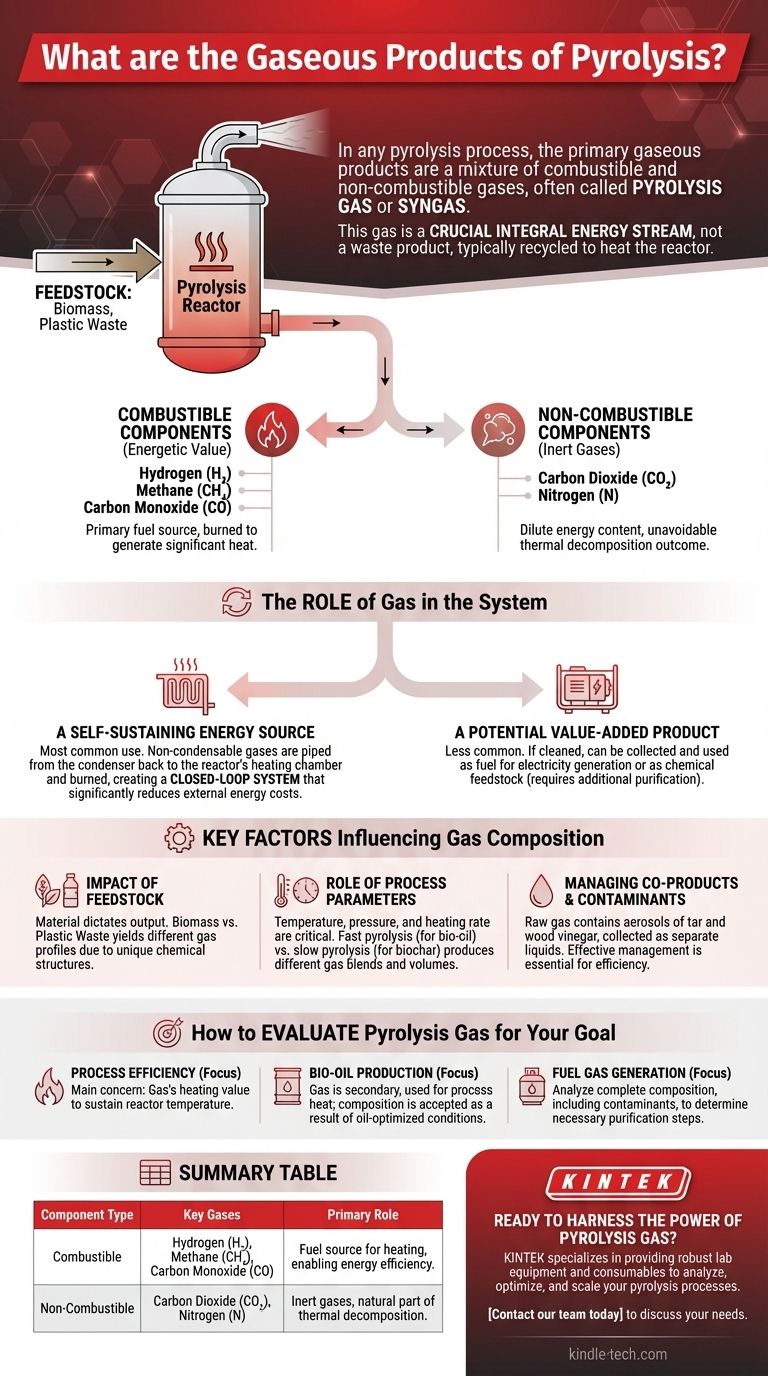
In any pyrolysis process, the primary gaseous products are a mixture of combustible and non-combustible gases. This mixture, often called pyrolysis gas or syngas, typically includes hydrogen (H₂), methane (CH₄), carbon monoxide (CO), carbon dioxide (CO₂), and various other hydrocarbons (CnHm). This gas is not a waste product; it is a crucial output that is most often captured and used to provide the thermal energy needed to sustain the pyrolysis reaction itself.
The critical insight is that the gaseous products of pyrolysis are not a secondary byproduct but an integral energy stream. While the exact composition varies, this gas stream's ability to be recycled to heat the reactor is fundamental to the energy efficiency and economic viability of most pyrolysis systems.

Understanding the Components of Pyrolysis Gas
Pyrolysis gas is a complex mixture whose composition depends heavily on the feedstock and process conditions. We can generally categorize its components into two main groups.
The Core Combustible Components
These are the gases that hold the energetic value. The primary combustible gases are hydrogen (H₂), methane (CH₄), and carbon monoxide (CO). These components are what make pyrolysis gas a viable fuel source, capable of being burned to generate significant heat.
The Inert and Non-Combustible Components
Alongside the valuable fuel gases, pyrolysis also produces non-combustible gases. These most commonly include carbon dioxide (CO₂) and nitrogen (N). Their presence dilutes the energy content of the gas mixture but is an unavoidable outcome of the thermal decomposition process.
The Role of Gas in the Pyrolysis System
Understanding what the gases are is only half the picture. Their role in the operational loop of the pyrolysis plant is what makes them so important.
A Self-Sustaining Energy Source
The most common and efficient use for pyrolysis gas is to power the very process that creates it. The non-condensable gases are piped from the condenser back to the reactor's heating chamber and burned. This creates a closed-loop system where the process generates its own fuel, significantly reducing external energy costs.
A Potential Value-Added Product
While less common, the pyrolysis gas can also be collected and stored. If cleaned and processed, it can be used as a fuel for other applications, such as powering generators for electricity or as a chemical feedstock, though this requires additional purification equipment.
Key Factors Influencing Gas Composition
The ratio of combustible to non-combustible gases is not fixed. It is a direct result of the feedstock being processed and the parameters of the operation.
The Impact of Feedstock
The material you put into the reactor dictates what comes out. The pyrolysis of biomass (like wood or agricultural waste) will produce a different gas profile than the pyrolysis of plastic waste. Each feedstock has a unique chemical structure that breaks down differently.
The Role of Process Parameters
Temperature, pressure, and heating rate are critical variables. For example, fast pyrolysis, which is optimized to produce liquid bio-oil, generates a specific blend of gases as a co-product. Slower pyrolysis processes designed to maximize solid biochar will yield a different gas composition and volume.
Managing Co-Products and Contaminants
The raw gas stream is not perfectly clean. It often contains aerosols of tar and wood vinegar, which are condensed and collected as separate liquid products during the cooling phase. Effectively managing these co-products is essential for both efficient system operation and maximizing the value of all outputs.
How to Evaluate Pyrolysis Gas for Your Goal
The "best" gas composition depends entirely on your primary objective. Understanding your goal helps clarify which aspects of the gas output are most important.
- If your primary focus is process efficiency: Your main concern is the gas's heating value, ensuring it's sufficient to sustain the reactor's temperature with minimal need for external fuel.
- If your primary focus is producing liquid bio-oil: The gas is a secondary product, and you will simply use it for process heat, accepting whatever composition results from the oil-optimized conditions.
- If your primary focus is generating a separate fuel gas: You must analyze the complete composition, including contaminants, to determine the necessary purification steps to make it suitable for its intended use.
Ultimately, the gaseous outputs of pyrolysis are a dynamic and valuable component of the process, not an afterthought.
Summary Table:
| Component Type | Key Gases | Primary Role in Pyrolysis |
|---|---|---|
| Combustible | Hydrogen (H₂), Methane (CH₄), Carbon Monoxide (CO) | Fuel source for heating the reactor, enabling energy efficiency. |
| Non-Combustible | Carbon Dioxide (CO₂), Nitrogen (N) | Inert gases that are a natural part of the thermal decomposition process. |
Ready to harness the power of pyrolysis gas in your operation? The composition and management of syngas are critical to the efficiency and economic viability of any pyrolysis system. At KINTEK, we specialize in providing robust lab equipment and consumables to help you analyze, optimize, and scale your pyrolysis processes. Whether you're focused on process efficiency, bio-oil production, or gas valorization, our experts can help you select the right tools for your specific feedstock and goals. Contact our team today to discuss how we can support your laboratory's pyrolysis research and development.
Visual Guide
Related Products
- Laboratory Test Sieves and Vibratory Sieve Shaker Machine
- Laboratory High Pressure Horizontal Autoclave Steam Sterilizer for Lab Use
- Custom PTFE Teflon Parts Manufacturer for PTFE Mesh F4 Sieve
- Custom PTFE Teflon Parts Manufacturer PTFE Beaker and Lids
- Hexagonal Boron Nitride HBN Spacer Cam Profile and Various Spacer Types
People Also Ask
- In which furnace is calcination and roasting done? A Guide to Selecting the Right Thermal Processing Equipment
- What are the negative effects of pyrolysis? High Costs and Environmental Risks Explained
- What is the temperature range for fast pyrolysis? Optimize Bio-Oil Yield from Biomass
- What are the uses of pyrolysis? Transform Waste into Energy, Fuel, and More
- How does a continuous furnace work? Unlock High-Volume, Consistent Thermal Processing
- Is pyrolysis self sufficient? How to Achieve Energy-Positive Waste Conversion
- What is the limitations of pyrolysis? Key Economic and Technical Challenges to Consider
- What are the various types of pyrolysis? Compare methods to optimize your biochar, bio-oil, or syngas yield.



















