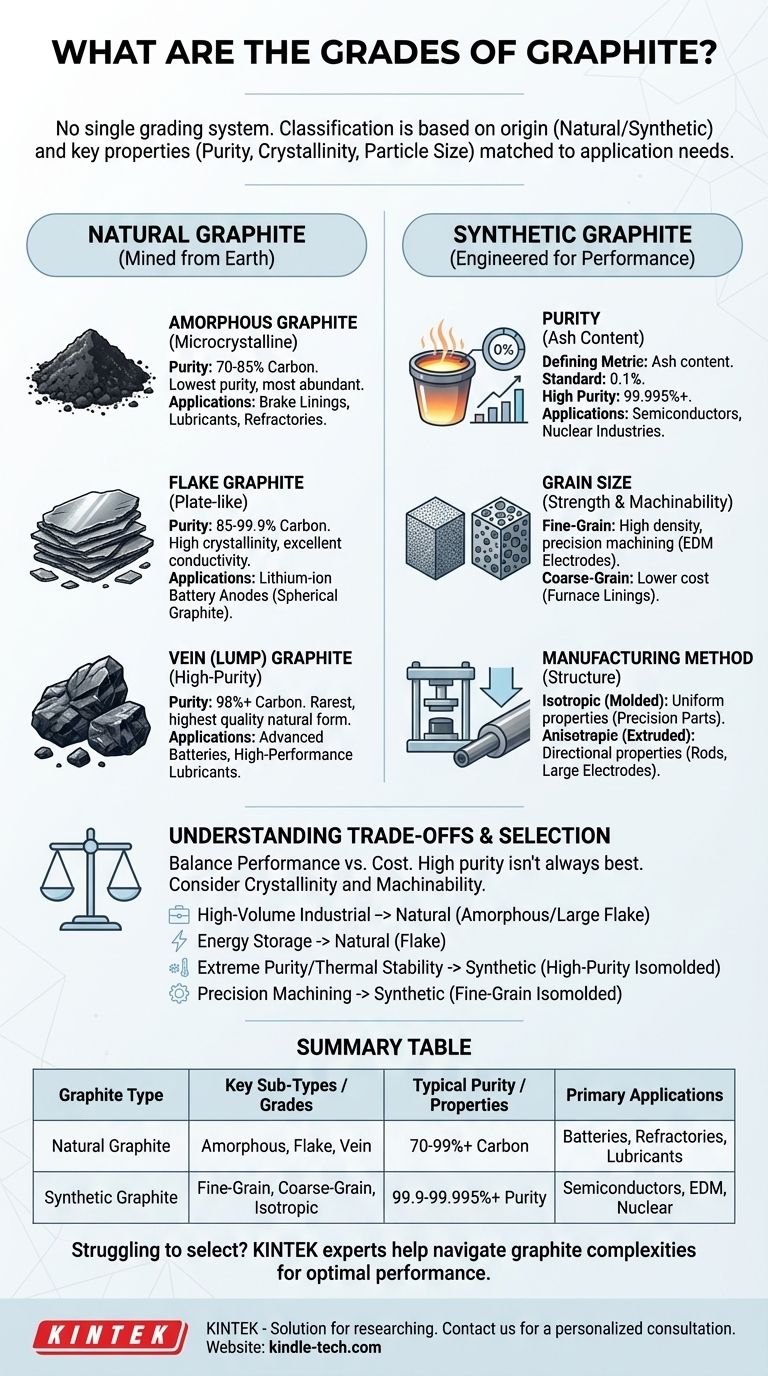
To be precise, there is no single, universally accepted grading system for graphite. Instead, a graphite's "grade" is a practical classification based on its origin—either natural or synthetic—and a set of key physical and chemical properties, primarily its purity (carbon content), crystallinity, and particle or grain size. These factors are defined by the specific requirements of its final application.
The most critical insight is to stop looking for a simple A-B-C grading chart. Instead, think of graphite selection as a process of matching your specific performance need—be it for batteries, refractories, or semiconductors—to a material defined by its source (natural/synthetic) and its measurable properties (purity, size, density).

The Two Fundamental Paths: Natural vs. Synthetic
The first and most important distinction in classifying graphite is its origin. This fundamental difference dictates its inherent properties, potential for purification, and ultimate cost.
Natural Graphite: Mined from the Earth
Natural graphite is a mineral formed through geological processes. It is mined and then processed to achieve the desired characteristics. Its properties are largely determined by its geological formation.
Synthetic Graphite: Engineered for Performance
Synthetic graphite is a man-made material produced by high-temperature treatment of carbonaceous raw materials like petroleum coke and coal tar pitch. This manufacturing process allows for extremely tight control over its final properties, enabling ultra-high levels of purity and specific structural forms.
Grading Natural Graphite
Natural graphite is typically categorized into three distinct commercial grades based on its morphology and crystallinity.
Amorphous Graphite: The Workhorse
Despite its name, amorphous graphite is not truly amorphous; it is microcrystalline. This is the lowest-purity and least crystalline form, with carbon content typically ranging from 70-85%. It is the most abundant and least expensive grade, making it ideal for high-volume industrial uses like brake linings, lubricants, and refractory materials used in steelmaking.
Flake Graphite: The Battery Standard
Flake graphite occurs as distinct, flat, plate-like particles with a high degree of crystallinity. Grades are determined by both carbon purity (85-99.9%) and flake size (jumbo, large, medium, fine). Its excellent electrical conductivity and crystalline structure make it the essential raw material for producing the spherical graphite used in lithium-ion battery anodes.
Vein (Lump) Graphite: The High-Purity Specialist
This is the rarest and highest-quality form of natural graphite, with purity often exceeding 98% in its raw state. It is mined from fissure veins and possesses superior thermal and electrical conductivity. Its rarity and high performance reserve it for specialty applications, including certain advanced battery types and high-performance lubricants.
Grading Synthetic Graphite: A Matter of Properties
Synthetic graphite isn't graded by type, but by a set of engineered properties that can be precisely controlled during manufacturing.
Purity (Ash Content): The Defining Metric
The most critical specification for synthetic graphite is its purity, measured by its ash content—the non-carbon impurities left after combustion. Standard grades may have 0.1% ash, while highly purified grades for the nuclear and semiconductor industries can achieve purities exceeding 99.995%.
Grain Size: Impact on Strength and Machinability
The size of the coke particles (grains) used in production dictates the material's final properties.
- Fine-grain graphite has high density and strength, allowing it to be machined to intricate shapes with smooth surface finishes, perfect for EDM electrodes and semiconductor crucibles.
- Coarse-grain graphite is less expensive and is typically used where fine detail is not required, such as in furnace linings and electrodes for steel production.
Manufacturing Method: Isotropic vs. Anisotropic
The forming method creates distinct structural properties.
- Extruded graphite is pushed through a die, aligning the grains and creating anisotropic properties (properties differ with and against the grain). It's cost-effective for rods and large electrodes.
- Isostatically-molded (Isomolded) graphite is pressed uniformly from all directions, creating an isotropic material with uniform properties. This is the highest-grade material, essential for applications requiring predictable performance and complex machining.
Understanding the Trade-offs
Choosing the right grade requires balancing performance needs with economic reality. The "best" graphite is rarely the one with the highest purity.
Why Not Always Use the Highest Purity?
Cost is the primary driver. The extensive energy and processing required to create high-purity synthetic graphite make it significantly more expensive than natural graphite. Using a 99.99% pure synthetic grade for a simple refractory application would be functionally effective but economically disastrous.
The Purity vs. Crystallinity Balance
Natural flake graphite can possess a highly ordered crystal structure that gives it excellent conductivity, sometimes rivaling synthetic grades. For applications like batteries, this high crystallinity is just as important as purity, making natural flake the ideal starting material.
Machinability and Application
An application requiring a complex, precision-machined part, like a semiconductor crystal-growing crucible, demands a fine-grain, isostatically-molded synthetic grade. The machinability and uniform thermal expansion of this grade are non-negotiable, making its high cost a necessary investment.
How to Select the Right Graphite Grade
Your selection should be dictated entirely by your end-use requirements.
- If your primary focus is high-volume industrial applications (refractories, steelmaking): Your choice will be driven by cost, favoring amorphous or large-flake natural graphite.
- If your primary focus is energy storage (lithium-ion batteries): You require the specific properties of high-purity spherical graphite, which is manufactured from natural flake graphite.
- If your primary focus is extreme purity and thermal stability (semiconductors, nuclear): High-purity, isostatically-molded synthetic graphite is your only viable option.
- If your primary focus is precision machining (EDM electrodes, molds): You need the strength and isotropic properties of a fine-grain, isostatically-molded synthetic graphite.
Understanding these core distinctions empowers you to look past simple labels and select the graphite with the precise properties your application demands.
Summary Table:
| Graphite Type | Key Sub-Types / Grades | Typical Purity / Properties | Primary Applications |
|---|---|---|---|
| Natural Graphite | Amorphous, Flake, Vein (Lump) | 70-99%+ Carbon | Batteries, Refractories, Lubricants |
| Synthetic Graphite | Fine-Grain, Coarse-Grain, Isotropic | 99.9-99.995%+ Purity | Semiconductors, EDM, Nuclear |
Struggling to select the perfect graphite material for your lab or production process? KINTEK specializes in high-performance lab equipment and consumables, including precision graphite components for applications from battery research to semiconductor manufacturing. Our experts can help you navigate the complexities of graphite grades to ensure optimal performance and cost-efficiency. Contact us today for a personalized consultation and discover the KINTEK difference in material science solutions.
Visual Guide
Related Products
- Carbon Graphite Plate Manufactured by Isostatic Pressing Method
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- Carbon Graphite Boat -Laboratory Tube Furnace with Cover
- Large Vertical Graphite Vacuum Graphitization Furnace
- Graphite Vacuum Continuous Graphitization Furnace
People Also Ask
- What is the function of graphite material when preparing Ga-LLZO sintered bodies? Ensure Sample Integrity in HIP
- What are the three types of coating? A Guide to Architectural, Industrial, and Special Purpose
- What are the advantages disadvantages and uses of sheet metal? The Ultimate Guide to Material Selection
- Does graphite lead electricity? Unlocking the Secrets of Its Atomic Structure
- What is the purpose of laminating? Protect and Enhance Your Documents for Long-Term Use



















