Determining the ash and mineral content of a material is a fundamental measure of its quality, composition, and purity. This simple analysis, which involves measuring the inorganic residue left after a sample is completely burned, provides critical insights into the non-combustible components. It serves as a vital checkpoint for ensuring materials meet required specifications and are free from unwanted inorganic contaminants.
Ash analysis is more than just measuring residue; it's a critical quality control tool that quantifies the total inorganic content—be it essential minerals, functional fillers, or harmful contaminants—to verify a product's integrity and composition against its design standards.

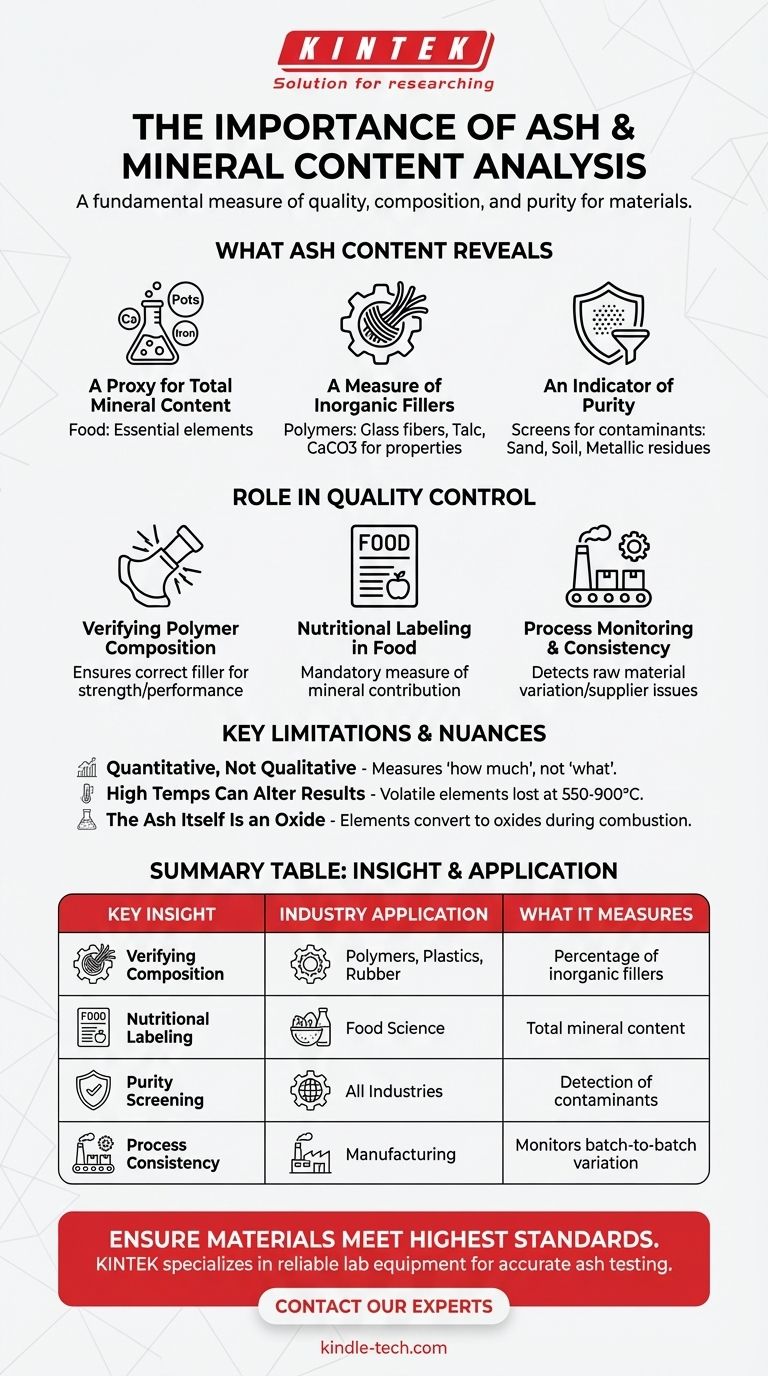
What Ash Content Actually Reveals
Ash testing is a process of thermal decomposition that separates the organic and inorganic portions of a sample. Understanding what this residue represents is key to interpreting the results.
A Proxy for Total Mineral Content
The "ash" is the inorganic, non-combustible portion of the sample. In fields like food science, this residue is a direct measure of the total mineral content—the calcium, potassium, iron, and other essential elements that do not burn away with the organic carbohydrates, fats, and proteins.
A Measure of Inorganic Fillers
In industrial materials like polymers, plastics, and rubber, inorganic compounds (fillers) are often added intentionally. These fillers, such as glass fibers, talc, or calcium carbonate, are used to modify the material's physical properties, like stiffness, strength, or to reduce cost. Ash analysis verifies that the correct percentage of this filler is present.
An Indicator of Purity
An ash test is also a simple and effective screening tool for contamination. Unexpectedly high ash content often signals the presence of inorganic impurities like sand, soil, dirt, or metallic residues from processing machinery.
The Role of Ash Analysis in Quality Control
Across various industries, ash content is a go-to metric for ensuring products meet their specifications. Its importance stems from its versatility as a checkpoint for composition, performance, and safety.
Verifying Material Composition in Polymers
For a plastic part designed for high strength, the percentage of reinforcing glass fiber is critical. Ash testing confirms this percentage. If the ash content is too low, the part may be too weak; if it's too high, it might be too brittle. This ensures the final product performs as engineered.
Nutritional Labeling in Food Science
The total ash content is a mandatory component of the proximate analysis for nutritional labeling on food products. It gives consumers a measure of the food's overall mineral contribution. It can also be a quality marker; for example, a high ash value in flour could indicate contamination from soil.
Process Monitoring and Consistency
For manufacturers using raw materials, monitoring the ash content of incoming batches is crucial. A sudden change in the ash value of a raw ingredient can signal a problem with a supplier or a change in the material's source, allowing for corrective action before it impacts the final product.
Understanding the Limitations and Nuances
While powerful, ash analysis is not a complete picture. Recognizing its limitations is essential for accurate interpretation.
It Is a Quantitative, Not Qualitative Test
A standard ash test tells you how much inorganic material is present, but it does not identify what specific minerals or elements are there. It cannot distinguish between a beneficial mineral like calcium and a contaminant like lead. For that, more advanced techniques like spectroscopy (e.g., ICP-OES) are required.
High Temperatures Can Alter the Results
Some inorganic elements can become volatile and be lost at the very high temperatures (typically 550-900°C) used in ashing. For instance, chlorides and some nitrates can vaporize, leading to a result that slightly underestimates the true total mineral content. The specific temperature and duration of the test method are critical for consistent and accurate results.
The Ash Itself Is an Oxide
The combustion process is one of oxidation. The residue left behind consists of oxides of the elements, not the elements in their original form. For example, calcium in a food sample will be converted to calcium oxide in the ash. This chemical change is an inherent part of the analysis.
Making the Right Choice for Your Goal
You can leverage ash analysis most effectively by aligning it with your specific objective.
- If your primary focus is material performance (e.g., plastics, rubber): Use ash content to verify the precise loading of inorganic fillers or reinforcements, ensuring the product meets its physical and mechanical specifications.
- If your primary focus is nutritional analysis: Use ash content as the standard measure for total mineral content for labeling purposes and as a first-line quality screen for raw ingredients.
- If your primary focus is process integrity and purity: Monitor ash content as a rapid and cost-effective indicator of inorganic contamination or variation in your supply chain.
Ultimately, ash analysis is a fundamental and powerful diagnostic tool for confirming that a material is, in fact, exactly what it is supposed to be.
Summary Table:
| Key Insight | Industry Application | What It Measures |
|---|---|---|
| Verifies Material Composition | Polymers, Plastics, Rubber | Percentage of inorganic fillers (e.g., glass fiber) |
| Nutritional Labeling | Food Science | Total mineral content for consumer information |
| Purity Screening | All Industries | Detection of inorganic contaminants (e.g., dirt, sand) |
| Process Consistency | Manufacturing | Monitors batch-to-batch variation in raw materials |
Ensure your materials meet the highest standards of quality and composition.
Ash and mineral content analysis is a cornerstone of effective quality control. Whether you are developing food products, engineering polymers, or monitoring raw materials, precise measurement is non-negotiable.
KINTEK specializes in providing reliable lab equipment and consumables for accurate ash testing and analysis. Our solutions help you verify filler percentages, ensure nutritional accuracy, and screen for contaminants with confidence.
Ready to enhance your quality control process? Contact our experts today to find the perfect analytical equipment for your laboratory's specific needs.
Visual Guide
Related Products
- 1800℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1400℃ Muffle Oven Furnace for Laboratory
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What is the process of dry ashing of sample treatment? A Guide to High-Temperature Mineral Analysis
- What is the difference between a muffle furnace and an oven? Choose the Right High-Temperature Tool
- What is the importance of muffle furnace in laboratory? Achieve Precise, Contaminant-Free Heating
- What is the difference between a muffle furnace and a blast furnace? Precision vs. Production
- What is the suitable material of construction of a muffle furnace? A Guide to High-Temperature Performance



















