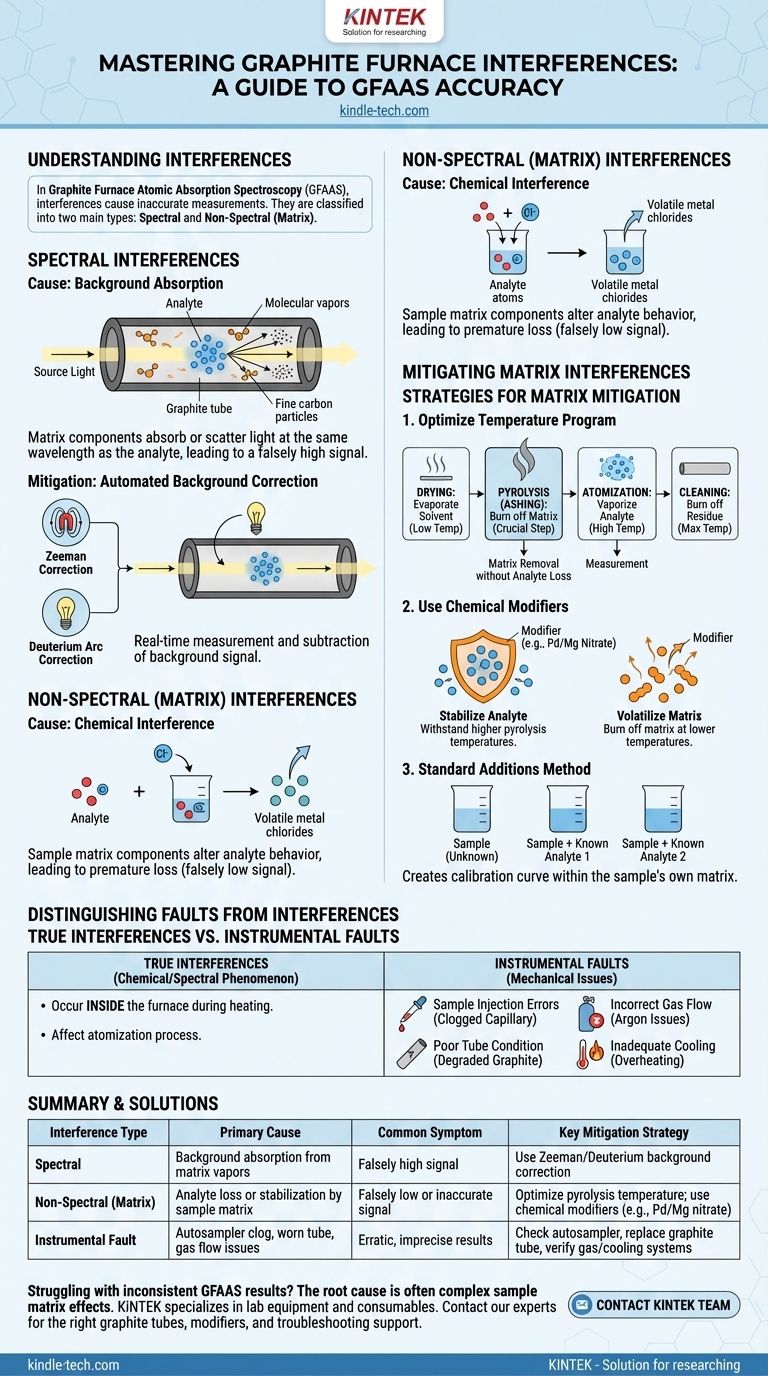
In graphite furnace analysis, interferences are effects that cause the measured signal for your target element to be inaccurate. They are broadly categorized into two main types: spectral interferences, where other atoms or molecules absorb light at the same wavelength, and non-spectral interferences (also called matrix effects), where other components in the sample alter the process of turning your element into a free atomic vapor.
The core challenge of graphite furnace atomic absorption (GFAAS) is not instrument failure, but managing the complex chemical and physical events inside the graphite tube. Success depends on separating the analyte from its surrounding matrix before the final measurement step.

The Two Core Types of Interference
Nearly every problem with GFAAS accuracy can be traced back to one of two fundamental sources of interference. Understanding the difference is the first step in troubleshooting.
Spectral Interferences
Spectral interferences occur when something other than the analyte absorbs or scatters light from the lamp, leading to a falsely high signal.
The primary cause is background absorption. As the sample matrix is heated to thousands of degrees, it can form molecular vapors (like alkali halides) or fine carbon particles that absorb or scatter the light.
Modern instruments almost completely eliminate this problem using automated background correction. The two most common and effective methods are Zeeman and Deuterium Arc correction, which measure and subtract the background absorption in real-time.
Non-Spectral (Matrix) Interferences
This is the more common and challenging category of interference in GFAAS. These are problems caused by the sample's matrix physically or chemically altering the behavior of the analyte during the heating process.
The most significant type is chemical interference. This happens when the analyte reacts with components of the matrix to form a highly stable compound. For example, chlorides in a sample can react with many elements to form volatile metal chlorides that are lost during the pre-heating (pyrolysis) step, before the final atomization measurement.
This premature loss of analyte results in a measurement that is falsely low.
How to Mitigate Matrix Interferences
Solving matrix interferences is the key to accurate GFAAS. The strategy involves optimizing the instrument's heating program and, when necessary, altering the sample's chemistry.
The Critical Role of the Temperature Program
The GFAAS heating cycle has distinct stages, and optimizing them is your primary tool for removing the matrix.
- Drying: Gently evaporates the solvent.
- Pyrolysis (or Ashing): This is the most crucial step. The temperature is raised high enough to burn off or vaporize the bulk of the sample matrix without losing the analyte.
- Atomization: The furnace is rapidly heated to a very high temperature to vaporize the analyte into a cloud of free atoms for measurement.
- Cleaning: The temperature is maxed out to burn off any remaining residue.
An effective pyrolysis step, where the matrix is removed before atomization, solves the majority of interference problems.
Using Chemical Modifiers
Sometimes, the matrix is too stable or the analyte is too volatile to be separated by temperature alone. In these cases, a chemical modifier is added to the sample.
Modifiers work in one of two ways:
- They stabilize the analyte: The modifier reacts with the analyte to form a compound that can withstand a higher pyrolysis temperature, allowing you to use more aggressive heating to remove the matrix.
- They make the matrix more volatile: The modifier reacts with the matrix to help it burn off at a lower temperature.
A common "universal" modifier is a mixture of palladium and magnesium nitrate, which stabilizes a wide range of elements.
The Standard Additions Method
When matrix effects are severe and cannot be eliminated, the method of standard additions can be used. This involves adding known quantities of the analyte to several aliquots of the sample itself. This creates a calibration curve within the sample's own matrix, effectively compensating for the specific interferences present.
Distinguishing Interferences from Instrumental Faults
While true interferences are chemical or spectral phenomena, many real-world problems produce similar symptoms but have mechanical causes.
True Interferences vs. System Problems
As discussed, interferences happen inside the furnace during the heating cycle, affecting the atomization process. Instrumental faults prevent the analysis from proceeding correctly in the first place.
Common Instrumental Issues
Before troubleshooting complex matrix chemistry, always check for simple mechanical failures.
- Sample Injection Errors: Issues with the autosampler, such as a clogged capillary tube due to sample crystallization, can prevent the correct volume of sample from being injected. This is a common cause of poor precision and low results.
- Poor Tube Condition: The graphite tube degrades with each use. An old or damaged tube leads to poor heating efficiency, memory effects (carryover from previous samples), and erratic results.
- Incorrect Gas Flow: The inert argon gas flow is critical for protecting the tube and sweeping away matrix vapors. Incorrect flow rates can cause high background signals and rapid tube degradation.
- Inadequate Cooling: The system relies on circulating cooling water. If the water pressure is too low or flow is blocked, the instrument can overheat, leading to unstable performance and damage.
A Practical Strategy for Troubleshooting
Use the symptoms of your problem to guide your investigation, starting with the simplest potential causes.
- If your primary focus is poor accuracy or low recovery: This strongly suggests a chemical matrix interference. Focus on optimizing the pyrolysis temperature and experiment with a chemical modifier like palladium nitrate.
- If your primary focus is high, unstable background signals: This suggests a spectral interference. Ensure your background correction system is active and effective, and that your pyrolysis step is sufficiently removing the bulk matrix.
- If your primary focus is erratic, imprecise results (poor reproducibility): This points toward an instrumental or physical problem. Before changing your method, inspect the autosampler capillary for clogs, check the condition of the graphite tube, and verify your cooling water and argon gas supplies.
Ultimately, achieving reliable GFAAS results is a systematic process of isolating and eliminating variables, from the mechanics of the instrument to the chemistry within the sample.
Summary Table:
| Interference Type | Primary Cause | Common Symptom | Key Mitigation Strategy |
|---|---|---|---|
| Spectral | Background absorption from matrix vapors | Falsely high signal | Use Zeeman/Deuterium background correction |
| Non-Spectral (Matrix) | Analyte loss or stabilization by sample matrix | Falsely low or inaccurate signal | Optimize pyrolysis temperature; use chemical modifiers (e.g., Pd/Mg nitrate) |
| Instrumental Fault | Autosampler clog, worn tube, gas flow issues | Erratic, imprecise results | Check autosampler, replace graphite tube, verify gas/cooling systems |
Struggling with inconsistent GFAAS results? The root cause is often complex sample matrix effects, not your instrument. KINTEK specializes in lab equipment and consumables, serving laboratory needs. Our experts can help you select the right graphite tubes and chemical modifiers, or provide troubleshooting support to achieve the precise, reliable data your research demands. Contact our team today to optimize your graphite furnace analysis!
Visual Guide
Related Products
- Graphite Vacuum Continuous Graphitization Furnace
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Large Vertical Graphite Vacuum Graphitization Furnace
People Also Ask
- What are the properties of graphite at high temperatures? Unlock Its Strength and Stability in Extreme Heat
- Is graphite affected by heat? Discover Its Remarkable Strength and Stability at High Temperatures
- What is the temperature resistance of graphite? Unlocking Its High-Temp Potential in Your Lab
- What is the maximum working temperature of graphite? Unlock High-Temp Performance with the Right Atmosphere
- How much temperature can graphite withstand? Unlock its true potential up to 3000°C



















