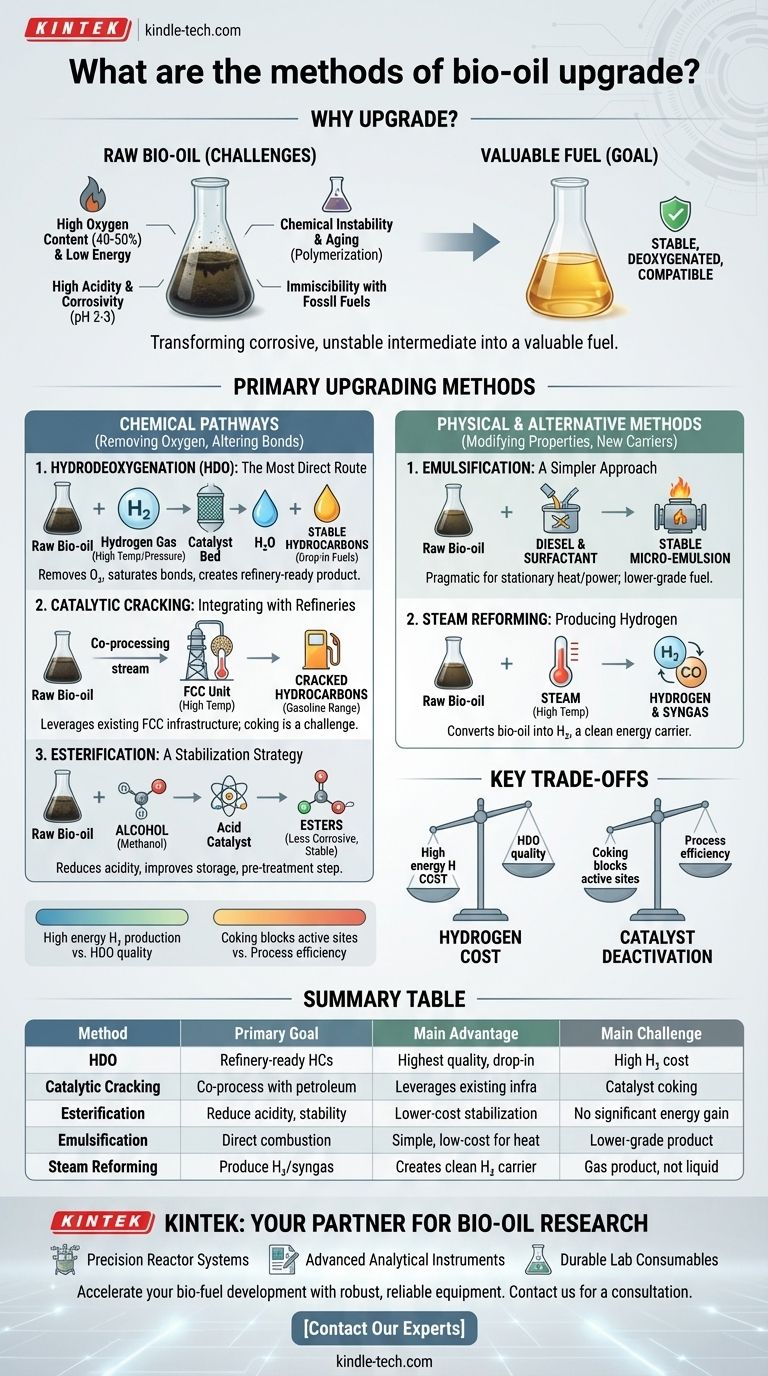
Upgrading raw bio-oil is not an option, but a necessity to transform it from a corrosive, unstable intermediate into a valuable fuel or chemical feedstock. The primary methods to achieve this are chemical processes like hydrodeoxygenation (HDO) and catalytic cracking, which remove oxygen and create stable hydrocarbons, and physical methods like emulsification, which prepare it for direct combustion.
The core challenge with raw bio-oil is its high oxygen and water content, which makes it acidic, unstable, and immiscible with conventional fuels. Therefore, every upgrading method is fundamentally a strategy for deoxygenation and stabilization to increase its value and compatibility with existing energy infrastructure.

Why Raw Bio-oil Requires Upgrading
Raw bio-oil, produced from the fast pyrolysis of biomass, is a complex mixture of water, char, and hundreds of oxygenated organic compounds. This unique composition presents several significant technical challenges that must be overcome before it can be widely used.
High Oxygen Content and Low Energy Value
The oxygen content of raw bio-oil can be as high as 40-50% by weight. This is the root cause of its low heating value, which is typically half that of conventional crude oil. Upgrading aims to remove this oxygen, thereby increasing the energy density of the final product.
Chemical Instability and Aging
Bio-oil is thermally unstable. Over time, or when heated, its reactive components (like aldehydes and ketones) polymerize, leading to a significant increase in viscosity. This "aging" process can turn the liquid oil into a solid sludge, creating major problems for storage and transport.
High Acidity and Corrosivity
The presence of organic acids, primarily acetic and formic acid, makes raw bio-oil highly acidic (pH 2-3). This level of acidity makes it corrosive to standard construction materials like carbon steel, requiring specialized and more expensive equipment for handling and processing.
Immiscibility with Fossil Fuels
The highly polar nature of bio-oil, due to its high oxygen and water content, makes it immiscible with non-polar hydrocarbon fuels like gasoline and diesel. This prevents it from being easily blended and co-processed in traditional petroleum refineries without pre-treatment.
Primary Chemical Upgrading Pathways
Chemical upgrading involves breaking and forming chemical bonds to fundamentally alter the composition of the bio-oil, primarily by removing oxygen.
Hydrodeoxygenation (HDO): The Most Direct Route
Hydrodeoxygenation (HDO), also known as hydrotreating, is the most studied and effective upgrading method. The bio-oil is reacted with hydrogen gas at high temperatures (300-400°C) and pressures over a catalyst.
The process removes oxygen in the form of water, saturates double bonds, and creates a stable, hydrocarbon-rich product. The resulting oil has a much higher heating value and resembles conventional crude oil fractions, making it suitable for further refining into "drop-in" fuels.
Catalytic Cracking: Integrating with Refineries
This approach involves introducing bio-oil into a Fluid Catalytic Cracker (FCC), a standard unit in most petroleum refineries. The high temperatures and catalyst in the FCC unit crack the large oxygenated molecules into smaller, more valuable gasoline-range hydrocarbons.
A common strategy is co-processing, where a small stream of bio-oil is fed into the FCC along with the primary petroleum gas oil stream. This leverages existing multi-billion dollar infrastructure but poses significant challenges related to catalyst deactivation and coke formation.
Esterification: A Stabilization Strategy
Esterification specifically targets the corrosive carboxylic acids in bio-oil. By reacting the oil with an alcohol (like methanol or ethanol) in the presence of an acid catalyst, the organic acids are converted into less corrosive and more stable esters.
This method primarily improves the storage stability and reduces the acidity of the bio-oil. However, it does not significantly increase the heating value, so it is often considered a pre-treatment step rather than a full upgrading solution.
Physical and Alternative Methods
These methods modify the physical properties of bio-oil or convert it into different energy carriers altogether, often with lower capital investment.
Emulsification: A Simpler Approach for Combustion
Emulsification is a physical blending process. Bio-oil is mixed with a hydrocarbon fuel (typically diesel) and a surfactant to create a stable micro-emulsion.
This allows the bio-oil to be burned in existing diesel engines, furnaces, or boilers with minimal modification. It is a pragmatic, low-cost path for using bio-oil for stationary heat and power generation but does not produce a high-grade transportation fuel.
Steam Reforming: Producing Hydrogen Instead of Fuel
Rather than converting bio-oil into a liquid fuel, steam reforming uses it as a feedstock to produce hydrogen or syngas (a mixture of hydrogen and carbon monoxide).
In this high-temperature process, the bio-oil reacts with steam to produce a gaseous product. This positions bio-oil not as a direct fuel substitute but as a renewable source for producing hydrogen, a critical industrial chemical and clean energy carrier.
Understanding the Trade-offs
No single upgrading method is perfect; each involves a balance of effectiveness, cost, and complexity.
The Hydrogen Dilemma
HDO is highly effective but relies on large quantities of high-pressure hydrogen. Producing this hydrogen is energy-intensive and expensive, representing a major operational cost and potential bottleneck for the economic viability of the process.
Catalyst Deactivation and Coking
Bio-oil is notoriously hard on catalysts. Its tendency to polymerize creates coke, a carbonaceous solid that deposits on the catalyst surface, blocking active sites and reducing its effectiveness. This rapid deactivation is a primary technical hurdle, especially for catalytic cracking.
Process Complexity vs. Product Quality
There is a direct relationship between the intensity of the upgrading process and the quality of the final product. Milder, less expensive methods like esterification or emulsification yield a lower-quality product with limited applications. In contrast, capital-intensive processes like HDO produce a high-quality, fungible hydrocarbon fuel.
Matching the Method to the Goal
The optimal upgrading strategy is dictated entirely by your end-product requirements and operational constraints.
- If your primary focus is producing drop-in transportation fuels: HDO is the most direct pathway to create a high-quality, refinery-ready hydrocarbon product, though it comes at a high capital and operational cost.
- If your primary focus is leveraging existing refinery infrastructure: Co-processing in an FCC unit offers a compelling route for integration, but significant research is still needed to overcome challenges with coking and catalyst stability.
- If your primary focus is stabilizing bio-oil for storage or local use: Esterification provides a targeted, lower-cost method to reduce corrosivity and prevent the oil from degrading during transport or storage.
- If your primary focus is immediate use in stationary engines or boilers: Emulsification offers the most pragmatic and cost-effective solution for using bio-oil as a substitute for heating oil or diesel in stationary power applications.
Ultimately, selecting the right bio-oil upgrading strategy depends on a clear-eyed assessment of your end-product requirements, available infrastructure, and economic constraints.
Summary Table:
| Method | Key Process | Primary Goal | Main Advantage | Main Challenge |
|---|---|---|---|---|
| Hydrodeoxygenation (HDO) | High-pressure H₂ with catalyst | Produce refinery-ready hydrocarbons | Highest quality, drop-in fuel potential | High hydrogen consumption and cost |
| Catalytic Cracking | Cracking in FCC unit with catalyst | Co-process with petroleum streams | Leverages existing refinery infrastructure | Catalyst deactivation from coking |
| Esterification | Reaction with alcohol and catalyst | Reduce acidity and improve stability | Lower-cost stabilization | Does not significantly increase energy value |
| Emulsification | Blending with diesel and surfactant | Enable direct combustion in engines/boilers | Simple, low-cost for heat/power | Lower-grade product, not for transport fuel |
| Steam Reforming | Reaction with steam at high temperature | Produce hydrogen/syngas | Creates a clean energy carrier (H₂) | Shifts product from liquid fuel to gas |
Ready to Upgrade Your Bio-Oil Process?
Navigating the complexities of bio-oil upgrading requires robust and reliable equipment. Whether you are developing a new catalytic process or scaling up an existing one, KINTEK is your trusted partner for high-performance laboratory solutions.
We provide the tools you need to succeed:
- Precision Reactor Systems for hydrodeoxygenation (HDO) and catalytic cracking experiments.
- Advanced Analytical Instruments to monitor product quality and catalyst performance.
- Durable Lab Consumables designed to handle the corrosive nature of raw bio-oil.
By partnering with KINTEK, you gain access to equipment that enhances the efficiency and accuracy of your research, helping you overcome challenges like catalyst deactivation and process optimization faster.
Let's discuss how our specialized lab equipment can accelerate your bio-fuel development. Contact our experts today for a personalized consultation!
Visual Guide
Related Products
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Anti-Cracking Press Mold for Lab Use
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- Laboratory Test Sieves and Sieving Machines
People Also Ask
- What role does an autoclave play in the acid treatment for microalgae disruption? Unlock High-Yield Cell Pretreatment
- What is the primary function of a laboratory autoclave in the pre-treatment of medical plastic waste for liquid fuel?
- What is the function of laboratory autoclaves in SCWR research? Predict Material Compatibility and Corrosion Kinetics
- What are the standard operating parameters for an autoclave? Master Temperature, Pressure, and Time for Sterilization
- What are the advantages of using an autoclave equipped with a stirring device for molten salt testing? Dynamic Accuracy