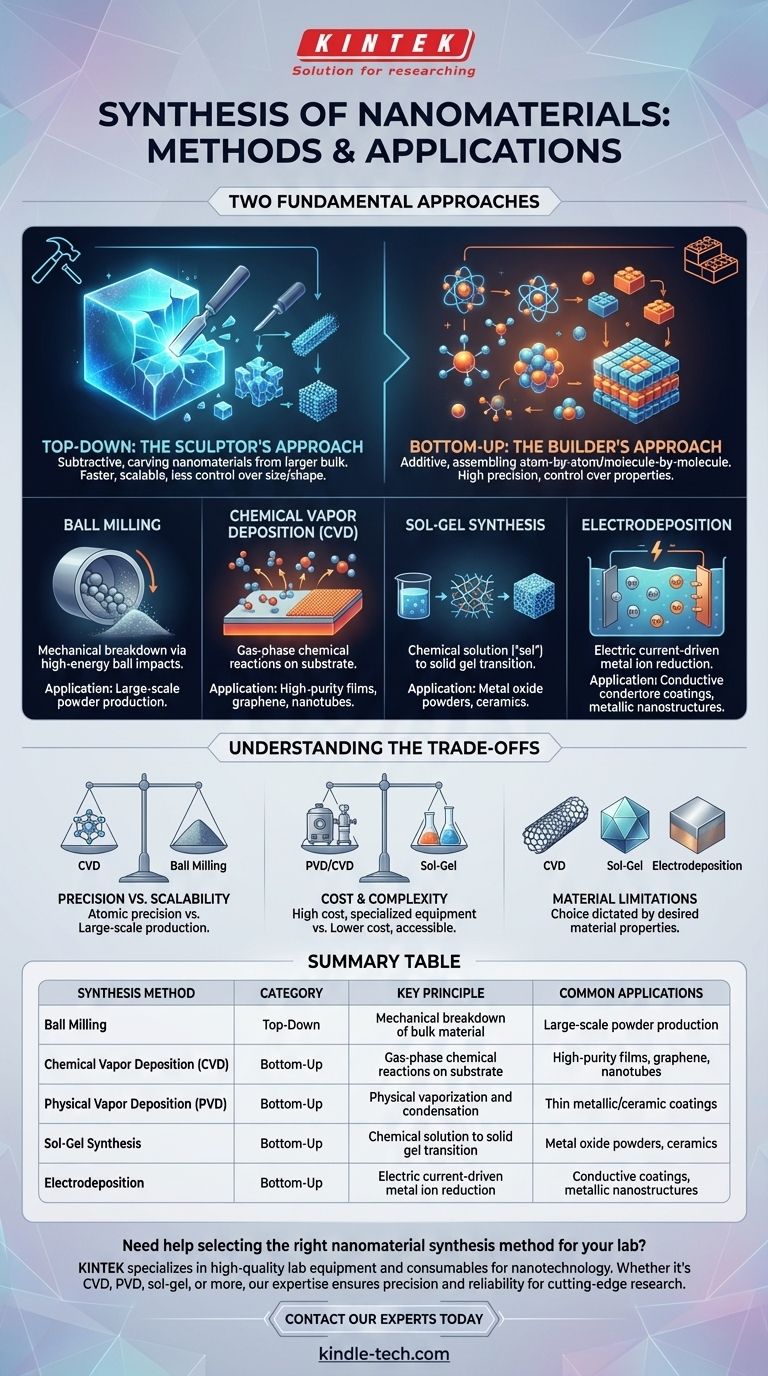
Fundamentally, nanomaterial synthesis methods are classified into two major categories: “top-down” and “bottom-up.” Top-down approaches are subtractive, carving nanomaterials from a larger bulk material, much like a sculptor carves a statue from a block of stone. In contrast, bottom-up methods are additive, assembling materials atom-by-atom or molecule-by-molecule from smaller components. The most common techniques you will encounter include chemical vapor deposition (CVD), physical vapor deposition (PVD), sol-gel synthesis, and ball milling.
The critical insight is not just knowing the names of the methods, but understanding the fundamental divide between them. Your choice between a "top-down" (carving) or "bottom-up" (building) approach will dictate the precision, cost, scale, and ultimate properties of your final nanomaterial.

The Two Fundamental Approaches: Top-Down vs. Bottom-Up
Every technique for creating nanomaterials falls into one of two strategic categories. Understanding this distinction is the key to navigating the field.
Top-Down Synthesis: The Sculptor's Approach
This approach involves the mechanical or chemical breakdown of a larger, bulk material to produce nanostructures. It is often faster and more suitable for large-scale production, but typically offers less control over the final particle size and shape.
Ball milling is a classic example of a top-down method. High-energy balls in a rotating chamber repeatedly collide with a bulk powder, progressively breaking the particles down to the nanoscale.
Other physical methods like laser ablation and arc-discharge also fit here. They use intense energy to vaporize a portion of a bulk target, with the vapor then condensing into nanoparticles.
Bottom-Up Synthesis: The Builder's Approach
This is the opposite strategy. It involves the controlled assembly of atoms, ions, or molecules to form nanostructures. This approach offers exceptionally high precision and control over the final material's properties.
Most advanced nanomaterial synthesis relies on bottom-up techniques because they allow for the design of materials with specific functions from the ground up.
Key Bottom-Up Synthesis Techniques
Bottom-up methods are diverse, but they are all based on the principle of controlled atomic or molecular assembly.
Chemical Vapor Deposition (CVD)
In CVD, a substrate is exposed to one or more volatile precursor gases. These gases react or decompose on the substrate's surface, leaving behind a high-quality, solid thin film or nanostructure.
This method is critical for producing high-purity materials like graphene and carbon nanotubes, where precise structural integrity is paramount.
Physical Vapor Deposition (PVD)
PVD describes a set of vacuum deposition methods where a material is physically transformed into a vapor, transported across a vacuum chamber, and condensed onto a substrate as a thin film.
Unlike CVD, this process does not involve chemical reactions. Common PVD techniques include sputtering (bombarding a target with ions) and thermal evaporation.
Sol-Gel Synthesis
The sol-gel method is a "wet-chemical" technique. It involves the evolution of a network of molecules from a chemical solution (the "sol") which, after a series of reactions, forms a gel-like solid phase.
This gel can then be processed (e.g., heated) to create dense ceramics, glasses, or metallic oxide powders. It is highly versatile and relatively low-cost.
Electrodeposition
Also known as electroplating, electrodeposition uses an electric current to reduce dissolved metal cations from a solution (an electrolyte). This causes them to form a coherent, thin metallic coating onto an electrode.
This technique provides excellent control over film thickness and morphology, making it ideal for creating conductive coatings and metallic nanostructures.
Understanding the Trade-offs
No single method is universally superior. The right choice is always a balance of competing factors.
Precision vs. Scalability
Bottom-up methods like CVD offer atomic-level precision but can be slow and difficult to scale for mass production.
Top-down methods like ball milling are highly scalable and can produce kilograms of material, but with much less control over particle size, distribution, and crystallinity.
Cost and Complexity
Vacuum-based methods like PVD and CVD require expensive, specialized equipment and controlled environments, making them high-cost.
Wet-chemical methods like sol-gel synthesis can often be performed with standard laboratory glassware, making them significantly cheaper and more accessible for certain materials like oxides.
Material and Structural Limitations
The choice of method is often dictated by the desired material. CVD is a go-to for carbon nanomaterials. Sol-gel is a workhorse for metal oxides. Electrodeposition is naturally limited to conductive materials.
Making the Right Choice for Your Goal
To select a method, you must first define your primary objective. The ideal technique is the one that best serves your end goal for the material.
- If your primary focus is high-purity, crystalline films or nanotubes: Vapor deposition methods like CVD or PVD are your most powerful tools.
- If your primary focus is large-scale production of powders or composites at low cost: Top-down milling or scalable chemical methods like sol-gel synthesis are the most practical choices.
- If your primary focus is depositing precise, thin metallic or conductive coatings: Electrodeposition and PVD offer excellent control and performance.
Ultimately, the synthesis method is not just a recipe; it is the tool you use to engineer the fundamental properties of matter.
Summary Table:
| Synthesis Method | Category | Key Principle | Common Applications |
|---|---|---|---|
| Ball Milling | Top-Down | Mechanical breakdown of bulk material | Large-scale powder production |
| Chemical Vapor Deposition (CVD) | Bottom-Up | Gas-phase chemical reactions on a substrate | High-purity films, graphene, nanotubes |
| Physical Vapor Deposition (PVD) | Bottom-Up | Physical vaporization and condensation | Thin metallic/ceramic coatings |
| Sol-Gel Synthesis | Bottom-Up | Chemical solution to solid gel transition | Metal oxide powders, ceramics |
| Electrodeposition | Bottom-Up | Electric current-driven metal ion reduction | Conductive coatings, metallic nanostructures |
Need help selecting the right nanomaterial synthesis method for your lab? KINTEK specializes in providing high-quality lab equipment and consumables for all your nanotechnology needs. Whether you're working with CVD, PVD, sol-gel, or other techniques, our expertise ensures you get the precision and reliability required for cutting-edge research. Contact our experts today to discuss how we can support your laboratory's success!
Visual Guide
Related Products
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- How does PACVD equipment improve DLC coatings? Unlock Low Friction and High Heat Resistance
- What is the hot filament chemical vapour deposition of diamond? A Guide to Synthetic Diamond Coating
- What machine is used to make lab-grown diamonds? Discover the HPHT & CVD Technologies
- How are reactants introduced into the reaction chamber during a CVD process? Mastering Precursor Delivery Systems
- What is the specific function of the metal filament in HF-CVD? Key Roles in Diamond Growth



















