In essence, pyrolysis operates by applying heat to a substrate in a closed, oxygen-free system. The specific operating conditions—primarily temperature, heating rate, and the duration the resulting gases spend in the hot zone—are not fixed but are deliberately manipulated to control the final products.
The core principle to understand is that pyrolysis is not a single process but a tunable one. By adjusting a few key variables, you can precisely control whether the process yields primarily solid char, liquid bio-oil, or combustible gas.

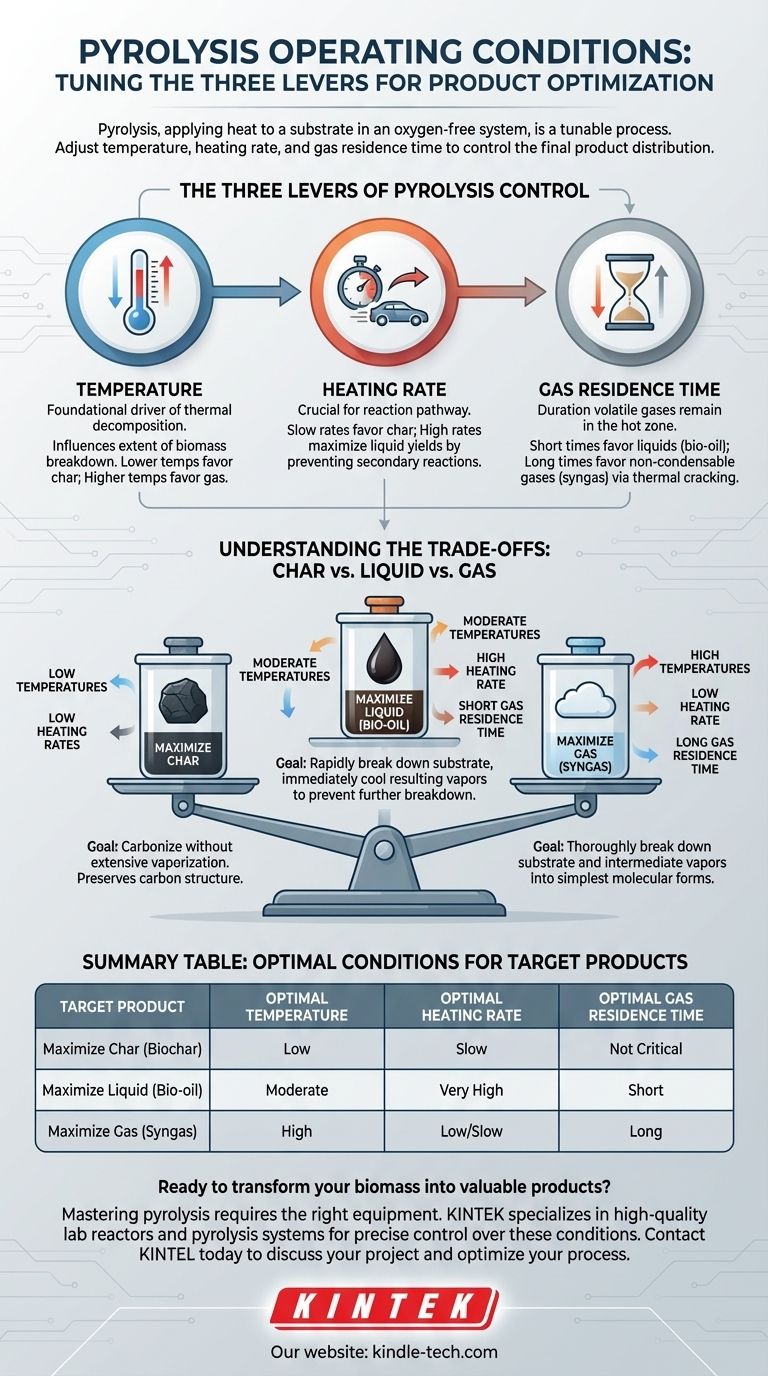
The Three Levers of Pyrolysis Control
To effectively manage a pyrolysis process, you must understand the impact of its three primary operating conditions. These variables work in concert to determine the final product distribution.
The Role of Temperature
Temperature is the foundational driver of thermal decomposition. The level of heat directly influences the extent to which the biomass breaks down.
Lower temperatures favor incomplete decomposition, leaving more solid carbon structures intact. Conversely, very high temperatures provide the energy needed to break down complex molecules into the simplest gaseous forms.
The Importance of Heating Rate
Heating rate refers to how quickly the substrate is brought to the target pyrolysis temperature. This variable is crucial for determining the primary reaction pathway.
A slow heating rate allows the material to break down in a more controlled, sequential manner, which tends to favor solid char formation. A very high heating rate causes rapid, almost explosive decomposition, which is essential for maximizing liquid yields by preventing secondary reactions.
The Impact of Gas Residence Time
Gas residence time is the amount of time the volatile gases, freshly released from the decomposing substrate, remain in the hot reactor.
A short residence time quickly removes these vapors from the heat, allowing them to be condensed into liquids. A long residence time keeps them in the hot zone, where they undergo further thermal cracking and reform into simpler, non-condensable gases.
Understanding the Trade-offs: Char vs. Liquid vs. Gas
The relationship between operating conditions and product yield is a set of trade-offs. Optimizing for one product category inherently means de-emphasizing the others.
How to Maximize Solid Char
To produce the maximum amount of char, the goal is to carbonize the material without extensive vaporization.
This is achieved with low temperatures and low heating rates. This combination drives off volatile components slowly while preserving the underlying carbon structure.
How to Maximize Liquid Products (Bio-oil)
To maximize liquid products, the process must rapidly break down the substrate and immediately cool the resulting vapors before they can break down further.
This requires average temperatures combined with a high heating rate and a short gas residence time. Reactor designs like ablative systems, which "melt" biomass on a hot surface, are built to facilitate this rapid heat transfer.
How to Maximize Gas
To produce the most gas, the objective is to thoroughly break down not only the original substrate but also the intermediate vapors into their simplest molecular forms.
This is best achieved with high temperatures, a low heating rate, and a long gas residence time, giving all components maximum time and energy to convert into permanent gases.
Making the Right Choice for Your Goal
Your desired output dictates the precise operating conditions you must implement. The process is fundamentally about balancing these variables to achieve a specific outcome.
- If your primary focus is producing biochar: Employ low temperatures and slow heating rates to favor solid carbon formation.
- If your primary focus is creating bio-oil: Use moderate temperatures, a very high heating rate, and ensure vapors are removed and cooled quickly.
- If your primary focus is generating syngas: Combine high temperatures with long gas residence times to allow for complete thermal cracking.
By mastering these conditions, you transform pyrolysis from a simple process into a precise manufacturing tool.
Summary Table:
| Target Product | Optimal Temperature | Optimal Heating Rate | Optimal Gas Residence Time |
|---|---|---|---|
| Maximize Char (Biochar) | Low | Slow | Not Critical |
| Maximize Liquid (Bio-oil) | Moderate | Very High | Short |
| Maximize Gas (Syngas) | High | Low/Slow | Long |
Ready to transform your biomass into valuable products?
Mastering pyrolysis requires the right equipment. KINTEK specializes in high-quality lab reactors and pyrolysis systems designed for precise control over temperature, heating rate, and residence time. Whether your goal is to produce biochar, bio-oil, or syngas, our experts can help you select the perfect solution for your laboratory's R&D or pilot-scale needs.
Contact KINTEL today to discuss your project and discover how our reliable lab equipment can optimize your pyrolysis process.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Graphite Vacuum Furnace IGBT Experimental Graphitization Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
People Also Ask
- What is the effect of temperature on calcination? Master Precise Heat Control for Material Properties
- What are the temperature stages of pyrolysis? Control Your Output from Biochar to Syngas
- What is a biomass pyrolysis plant? Turn Waste into Renewable Energy & Biochar
- What is the primary function of an industrial rotary tube furnace? Master Tungsten Powder Hydrogen Reduction
- What is the process of wood pyrolysis? A Guide to Converting Wood into Biochar, Bio-oil, and Syngas
- How do you choose calcination temperature? A Guide to Optimizing Material Properties
- What are the uses of pyrolysis? Transform Waste into Energy, Fuel, and More
- Is pyrolysis destructive? Unlocking Value from Waste Through Controlled Thermal Transformation



















