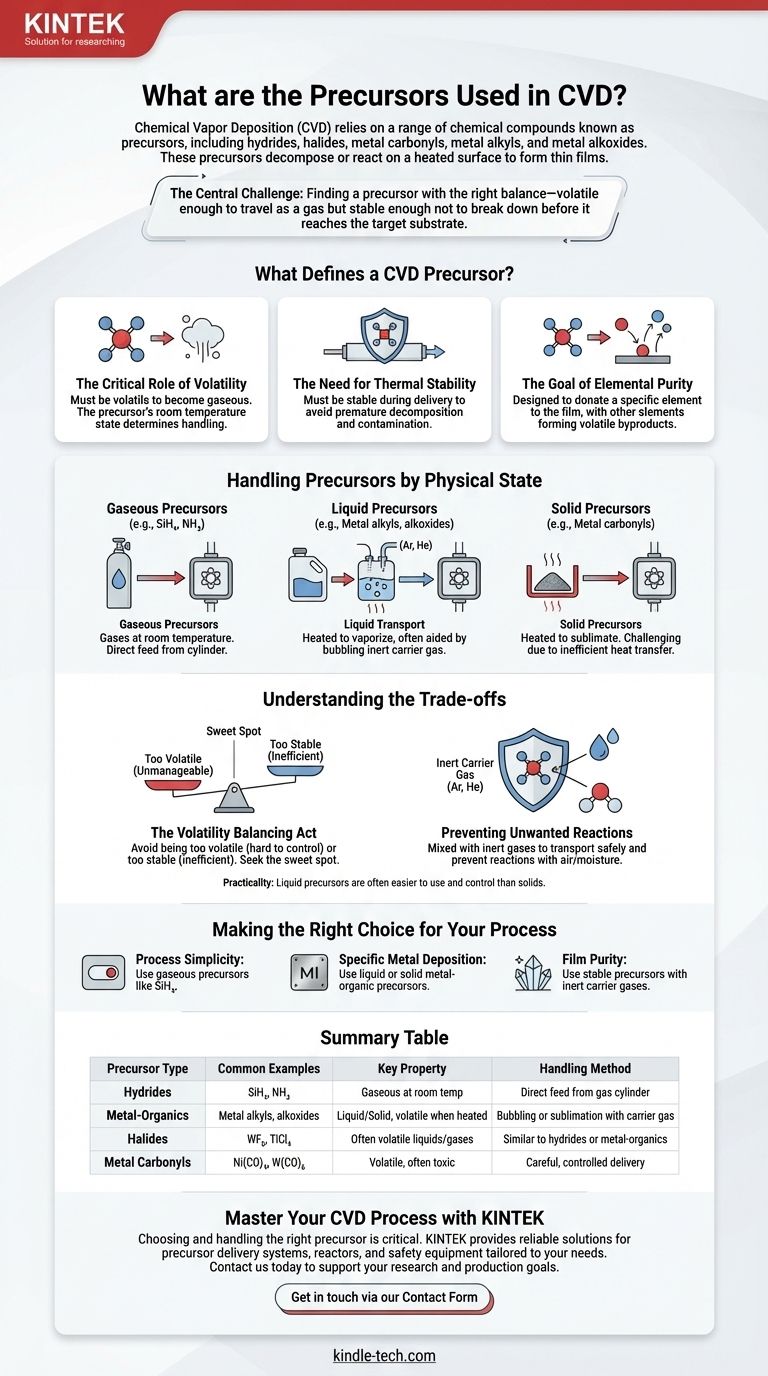
In short, Chemical Vapor Deposition (CVD) relies on a range of chemical compounds known as precursors, which include categories like hydrides (e.g., SiH₄, NH₃), halides, metal carbonyls, metal alkyls, and metal alkoxides. These precursors are the source materials that decompose or react on a heated surface to form the desired thin film.
The central challenge in CVD is not just finding a chemical that contains the element you want to deposit, but finding a precursor with the right balance of properties. The ideal precursor must be volatile enough to travel as a gas but stable enough not to break down before it reaches the target substrate.

What Defines a CVD Precursor?
To understand why specific chemicals are used in CVD, we must look at the fundamental properties required to successfully transport a material and deposit it as a high-quality film.
The Critical Role of Volatility
A precursor must be volatile, meaning it can be readily converted into a gaseous state. This is non-negotiable, as the "vapor" in Chemical Vapor Deposition refers to the gaseous precursor.
The physical state of the precursor at room temperature—solid, liquid, or gas—determines how it is handled to achieve this vapor phase.
The Need for Thermal Stability
While a precursor must be volatile, it must also be stable enough to be delivered to the reactor chamber without decomposing prematurely.
If the compound breaks down in the delivery lines, it can cause contamination and will never reach the substrate to form the intended film.
The Goal of Elemental Purity
An effective precursor is designed to donate a single, specific element to the film.
Other elements within the precursor molecule are engineered to form volatile byproducts during the reaction. These byproducts are then exhausted from the chamber, leaving behind a pure or near-pure film.
Handling Precursors by Physical State
The method for delivering the precursor to the CVD reactor depends entirely on its natural state.
Gaseous Precursors
Precursors that are gases at room temperature are the simplest to handle. They can be precisely controlled and fed directly into the reactor from a cylinder under normal pressure conditions.
Liquid Precursors
Liquid precursors require an additional step. They must be heated to generate a vapor, a process often aided by bubbling an inert carrier gas (like argon or helium) through the liquid. This gas mixture is then transported to the reactor.
Solid Precursors
Solid precursors present the most significant handling challenges. They must be heated to sublimate (turn directly into a gas), but this is often inefficient due to their smaller surface area and poor heat transfer compared to liquids.
Understanding the Trade-offs
Selecting and using a precursor involves balancing competing properties and managing potential risks. Failing to understand these trade-offs leads to poor film quality and failed deposition runs.
The Volatility Balancing Act
A precursor cannot be too volatile. If it evaporates too easily, it can be difficult to store and control. The material might evaporate before it can even be delivered properly to the vacuum chamber.
The goal is a "sweet spot"—volatile enough to vaporize under controlled conditions, but not so volatile that it becomes unmanageable.
Preventing Unwanted Reactions
Precursors can be sensitive and may react with air or moisture, leading to degradation and contamination.
To prevent this, they are often mixed with inert carrier gases like argon (Ar) or helium (He). These gases safely transport the precursor vapor to the substrate without participating in unwanted side reactions like oxidation.
The Practicality of Liquid vs. Solid
While both require heating, liquid precursors are generally considered easier to use than solids. Their ability to flow allows for more consistent vaporization and better thermal management, leading to more repeatable process control.
Making the Right Choice for Your Process
Your choice of precursor handling strategy is dictated by the material you need to deposit and the complexity you are willing to manage.
- If your primary focus is process simplicity: Gaseous precursors like silane (SiH₄) are the most straightforward as they require minimal preparation.
- If you need to deposit a specific metal: You will likely use a liquid or solid metal-organic precursor, which requires a carefully designed heating and vapor delivery system.
- If your primary focus is film purity: You must use a stable precursor and an inert carrier gas to prevent degradation and ensure only the desired reaction occurs at the substrate.
Ultimately, selecting the right precursor and mastering its delivery is fundamental to controlling the quality and properties of the final deposited film.
Summary Table:
| Precursor Type | Common Examples | Key Property | Handling Method |
|---|---|---|---|
| Hydrides | SiH₄, NH₃ | Gaseous at room temperature | Direct feed from gas cylinder |
| Metal-Organics | Metal alkyls, alkoxides | Liquid or solid, volatile when heated | Bubbling or sublimation with carrier gas |
| Halides | WF₆, TiCl₄ | Often volatile liquids or gases | Similar to hydrides or metal-organics |
| Metal Carbonyls | Ni(CO)₄, W(CO)₆ | Volatile, but often toxic | Requires careful, controlled delivery |
Master Your CVD Process with KINTEK
Choosing and handling the right precursor is critical for achieving high-purity, uniform thin films. Whether you are working with gaseous, liquid, or solid precursors, KINTEK's expertise in lab equipment and consumables can help you optimize your deposition process.
We provide reliable solutions for precursor delivery systems, reactors, and safety equipment tailored to your laboratory's specific needs. Contact us today to discuss how we can support your research and production goals.
Get in touch via our Contact Form to speak with an expert!
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- CVD Diamond Domes for Industrial and Scientific Applications
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- CVD Diamond Cutting Tool Blanks for Precision Machining
People Also Ask
- What is the plasma CVD process? Achieve Low-Temperature Thin Film Deposition
- What is plasma CVD? Unlock Low-Temperature Thin Film Deposition for Sensitive Materials
- How does plasma vapor deposition work? A Low-Temperature Coating Solution for Sensitive Materials
- How does RF power create plasma? Achieve Stable, High-Density Plasma for Your Applications
- How does PECVD work? Enable Low-Temperature, High-Quality Thin Film Deposition



















