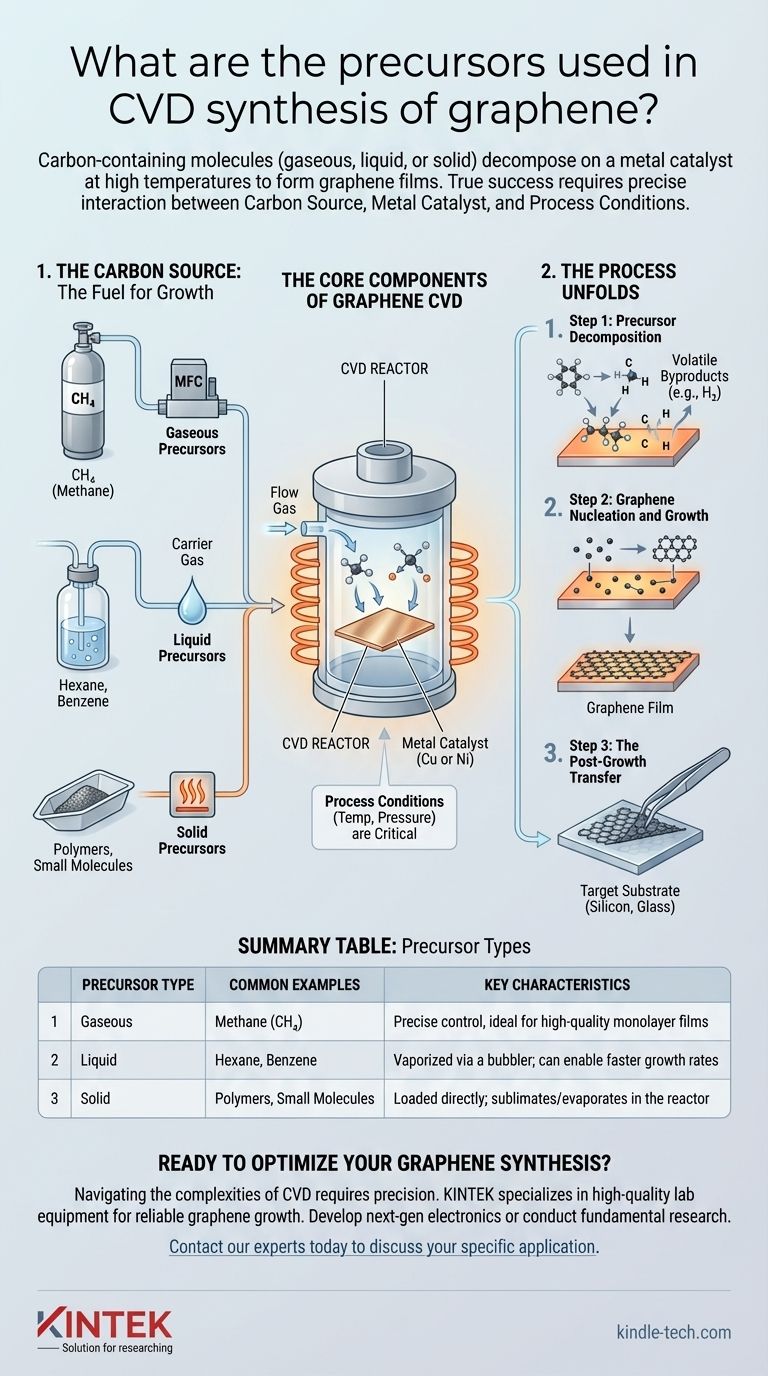
The precursors for graphene synthesis via Chemical Vapor Deposition (CVD) are carbon-containing molecules that can exist in gaseous, liquid, or solid states. Gaseous sources like methane (CH4) are the most common, but liquid precursors such as hexane and various solid carbon sources can also be used. These precursors are introduced into a high-temperature reactor where they decompose on a metal catalyst to form the graphene film.
The specific carbon precursor is only one piece of the puzzle. True success in graphene CVD depends on the precise interaction between three critical components: the carbon source, a metal catalyst, and carefully controlled process conditions like temperature and pressure.

The Core Components of Graphene CVD
CVD is a "bottom-up" synthesis technique, meaning you build the graphene atom-by-atom from a chemical source. This requires a well-defined recipe with several key ingredients working in concert.
The Carbon Source: The Fuel for Growth
The precursor is the molecule that provides the carbon atoms for the graphene lattice. These sources are categorized by their physical state.
Gaseous precursors, most notably methane (CH4), are widely used due to the precise control they offer over delivery into the reactor via mass flow controllers.
Liquid precursors, like hexane, are vaporized in a device called a bubbler. A carrier gas is passed through the liquid, becomes saturated with its vapor, and transports it into the reaction chamber.
Solid precursors are loaded directly into the reactor. They must be heated to sublimate or evaporate, turning into a gas that can participate in the reaction.
The Metal Catalyst: The Crucial Workbench
A transition metal substrate, typically a thin foil of copper (Cu) or nickel (Ni), is essential. It is not just a surface for growth; it is an active catalyst.
The catalyst's primary role is to lower the energy barrier required to break down the precursor molecules. At high temperatures, the hydrocarbon precursor decomposes into active carbon radicals on the metal's surface.
The choice of metal also dictates the growth mechanism and ultimately influences the quality and number of graphene layers produced.
Carrier Gases and Environment: The Delivery System
Inert or reducing gases, such as Argon (Ar) and Hydrogen (H2), serve as carrier gases. Their function is to transport the precursor molecules through the hot zone of the reactor to the catalyst surface.
The entire process occurs in a sealed reactor at very high temperatures, often approaching 1000°C. This controlled atmospheric environment is critical for the chemical reactions to proceed correctly.
How the Process Unfolds
Understanding the sequence of events inside the CVD reactor clarifies the role of each component.
Step 1: Precursor Decomposition
Gaseous hydrocarbon precursors are fed into the reactor. As they pass over the heated metal catalyst, the high temperature and the catalytic activity of the metal surface cause the precursor molecules to break apart, or decompose.
This decomposition releases carbon atoms or small carbon radicals, while other elements (like hydrogen from methane) are eventually removed as volatile byproducts.
Step 2: Graphene Nucleation and Growth
The liberated carbon atoms adsorb onto and diffuse across the metal surface. They begin to link together, forming the characteristic hexagonal lattice structure of graphene.
This process starts at multiple "nucleation" sites and the small graphene islands grow until they merge, ideally forming a continuous, single-atom-thick sheet across the entire substrate.
Step 3: The Post-Growth Transfer
Because graphene is often grown on an opaque metal foil, a final step is usually required. The graphene film must be carefully detached from the metal catalyst and transferred to a target substrate, such as a silicon wafer or glass, to be used in electronic or optical applications.
Understanding the Trade-offs
While CVD is a powerful method for producing high-quality graphene, it is not without its challenges. The process involves a delicate balance of competing factors.
Precursor Choice vs. Graphene Quality
The choice of precursor is critical. Simple molecules like methane offer excellent control and generally lead to higher-quality, monolayer graphene. More complex liquid or solid precursors can enable faster growth but may also introduce more defects into the film.
The Challenge of Uniformity
Achieving a perfectly uniform, large-area, single-layer graphene sheet is exceptionally difficult. Variations in temperature, gas flow, or catalyst surface quality can lead to the formation of multi-layer patches, wrinkles, and grain boundaries, which can affect the material's performance.
The Catalyst's Double-Edged Sword
The catalyst is essential for the reaction, but it can also be a source of problems. Impurities on the catalyst surface can disrupt growth, and the grain structure of the metal foil itself can be imprinted onto the graphene film, creating defects.
Making the Right Choice for Your Goal
The optimal CVD parameters depend entirely on the desired outcome. Your choice of precursor and process should be guided by your specific application.
- If your primary focus is large-area, high-quality films for electronics: Methane is the industry standard precursor, typically paired with a high-purity copper foil catalyst to favor monolayer growth.
- If your primary focus is rapid synthesis or fundamental research: Exploring liquid or solid precursors can offer new insights into growth kinetics and may provide pathways to novel carbon nanostructures.
- If your primary focus is process repeatability and control: Prioritize high-purity gaseous precursors with precise mass-flow control systems and invest in meticulous characterization of your catalyst substrates.
Mastering graphene synthesis is about understanding and controlling the interplay of these fundamental components to reliably produce the desired material.
Summary Table:
| Precursor Type | Common Examples | Key Characteristics |
|---|---|---|
| Gaseous | Methane (CH₄) | Precise control, ideal for high-quality monolayer films |
| Liquid | Hexane, Benzene | Vaporized via a bubbler; can enable faster growth rates |
| Solid | Polymers, Small Molecules | Loaded directly; sublimates/evaporates in the reactor |
Ready to Optimize Your Graphene Synthesis?
Navigating the complexities of CVD—from precursor selection to catalyst optimization—requires precision equipment and expert support. KINTEK specializes in providing the high-quality lab equipment and consumables you need for reliable, repeatable graphene growth.
Whether you are developing next-generation electronics or conducting fundamental research, we can help you achieve your goals. Contact our experts today to discuss your specific application and discover how our solutions can enhance your lab's capabilities.
Visual Guide
Related Products
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
- CVD Diamond Domes for Industrial and Scientific Applications
- CVD Diamond Cutting Tool Blanks for Precision Machining
People Also Ask
- How many types of physical Vapour deposition are there? A Guide to Evaporation vs. Sputtering
- What is the purpose of N2 and O2 flow meters in deposition? Master Film Stoichiometry and Material Performance
- What is the procedure of magnetron sputtering? A Step-by-Step Guide to Thin Film Deposition
- What are the different types of thin film coatings? A Guide to Deposition Methods & Materials
- Is A CVD diamond a real diamond? Discover the Truth About Lab-Grown Diamonds
- What is the low temperature for graphene growth? Unlock Scalable, Cost-Effective Production
- What is chemical vapor deposition of carbon? A Guide to Growing Advanced Materials
- What is the temperature range for LPCVD? A Guide to Process Parameters by Material



















