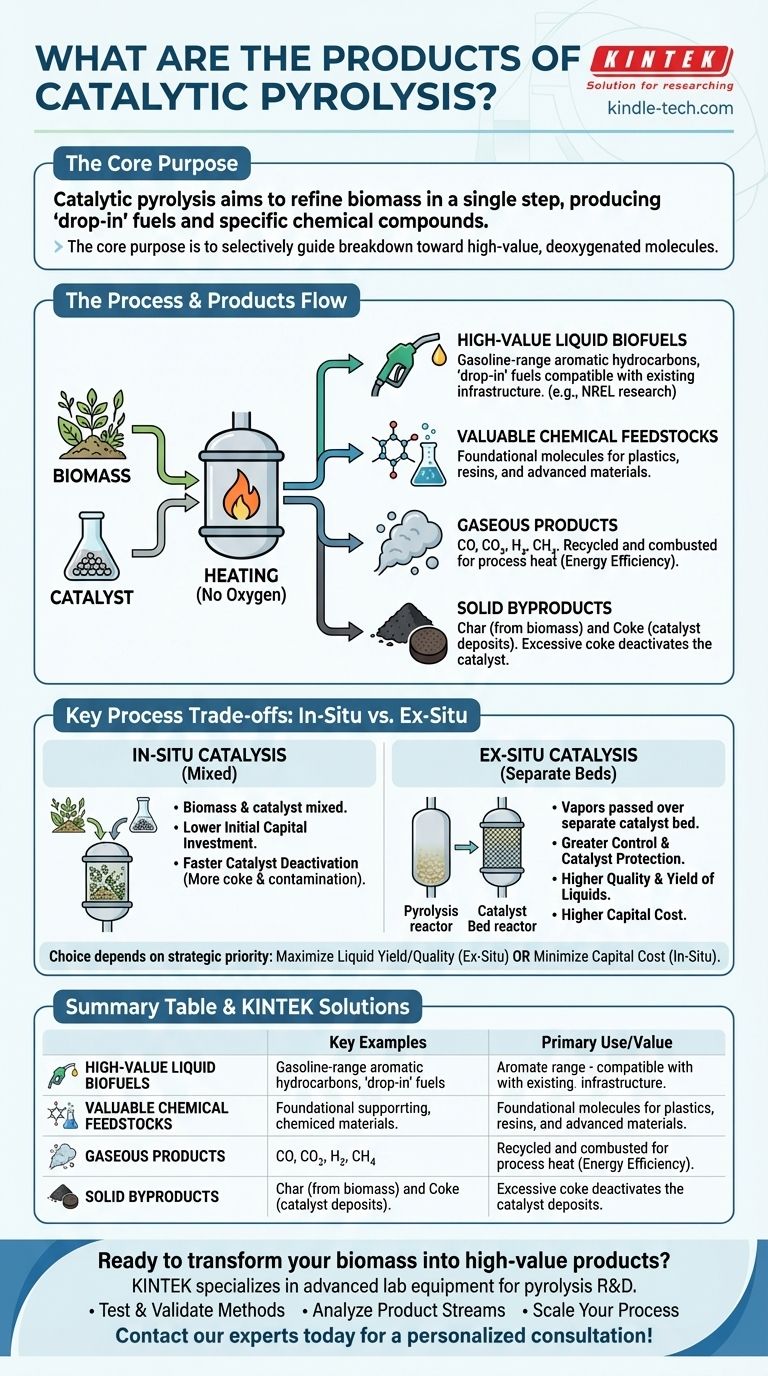
The primary products of catalytic pyrolysis are upgraded liquid biofuels, valuable chemical feedstocks, and various byproducts like gases and solid char. Unlike standard pyrolysis which creates a crude bio-oil requiring significant post-processing, catalytic pyrolysis aims to refine the material in a single step, producing "drop-in" fuels like gasoline or specific chemical compounds.
The core purpose of catalytic pyrolysis is not just to break down biomass, but to selectively guide that breakdown toward high-value, deoxygenated molecules that are more stable and immediately useful than the products of traditional pyrolysis.

Understanding the Process and Its Outputs
Catalytic pyrolysis is an advanced thermochemical conversion process. It involves heating organic material, such as biomass, in the complete absence of oxygen and in the presence of a catalyst. The catalyst is the key differentiator, upgrading the pyrolysis vapors as they form.
High-Value Liquid Biofuels
The most sought-after products are liquid hydrocarbons that are compatible with existing fuel infrastructure. The catalyst actively removes oxygen and cracks large organic molecules into smaller, more desirable fuel-range molecules.
A key example is gasoline-range aromatic hydrocarbons. Research, such as that by NREL, has focused on producing finished fuels that could be sold at a competitive price, demonstrating the potential for creating true "drop-in" biofuels.
Valuable Chemical Feedstocks
Beyond fuel, the process can be tuned to produce specific chemical building blocks. These are the foundational molecules used to create plastics, resins, and other advanced materials. By selecting the right catalyst and conditions, the process can maximize the yield of these high-value chemicals.
Gaseous Products
Like any pyrolysis process, a stream of non-condensable gases is also produced. This gas mixture typically includes carbon monoxide (CO), carbon dioxide (CO2), hydrogen (H2), and light hydrocarbons like methane. This gas is often recycled and combusted to provide the necessary heat for the pyrolysis reactor, improving the overall energy efficiency of the system.
Solid Byproducts: Char and Coke
Two solid products result from the process. Char is the carbon-rich solid residue left over from the biomass itself.
More critically, coke is a form of carbon that deposits on the surface of the catalyst during the chemical reactions. While char is an expected byproduct, excessive coke formation is problematic as it deactivates the catalyst, reducing its effectiveness and lifespan.
Key Process Trade-offs: In-Situ vs. Ex-Situ
The method used to introduce the catalyst has a profound impact on the process efficiency and the final product yields. This is the central design choice in building a catalytic pyrolysis system.
In-Situ Catalysis (Mixed)
In this approach, the biomass and catalyst are mixed together in a single reactor. The primary advantage is a lower initial capital investment due to the simpler, single-reactor design.
However, this method leads to faster catalyst deactivation. Direct contact with the biomass solids and primary vapors leads to rapid coke formation and contamination, requiring more frequent catalyst regeneration or replacement.
Ex-Situ Catalysis (Separate Beds)
Here, the biomass is pyrolyzed in one reactor, and the resulting vapors are immediately passed over a separate, dedicated catalyst bed in a second reactor. This dual-bed system offers greater control and protects the catalyst.
By separating the stages, the catalyst is exposed only to vapors, not solids, which significantly reduces coke formation and extends its operational life. While this approach has a higher capital cost, it often results in a higher quality and yield of the desired liquid products.
How to Apply This to Your Goal
The choice between catalytic pyrolysis methods depends entirely on the strategic priority of the project.
- If your primary focus is on maximizing the yield and quality of liquid fuels: Choose an ex-situ design to protect the catalyst and optimize the upgrading conditions.
- If your primary focus is on minimizing initial capital costs for a smaller-scale operation: An in-situ design offers a simpler and more direct pathway, though with higher operational challenges.
Ultimately, catalytic pyrolysis represents a targeted technology designed to transform low-value biomass into high-value, ready-to-use liquid products.
Summary Table:
| Product Type | Key Examples | Primary Use/Value |
|---|---|---|
| Liquid Biofuels | Gasoline-range hydrocarbons, 'drop-in' fuels | Direct replacement for fossil fuels, energy |
| Chemical Feedstocks | Aromatics, olefins | Building blocks for plastics, resins, materials |
| Gaseous Products | CO, CO₂, H₂, CH₄ | Recycled for process heat, improving energy efficiency |
| Solid Byproducts | Char (from biomass), Coke (on catalyst) | Char as soil amendment; Coke deactivates catalyst |
Ready to transform your biomass into high-value products?
KINTEK specializes in advanced lab equipment for pyrolysis research and development. Whether you are exploring catalyst performance, optimizing reaction conditions for bio-oil yield, or scaling up from bench to pilot, our reactors and analytical tools are designed for precision and reliability.
We help you:
- Test and validate different catalytic pyrolysis methods (in-situ vs. ex-situ).
- Analyze product streams to maximize yields of biofuels and chemical feedstocks.
- Scale your process with equipment tailored to your specific R&D goals.
Let's discuss how our solutions can accelerate your bioenergy or biorefining project. Contact our experts today for a personalized consultation!
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
People Also Ask
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas
- What are the different types of pyrolysis machines? Choose the Right System for Your Output
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success
- What are the conditions for biomass pyrolysis? Optimize Temperature, Heating Rate & Time



















