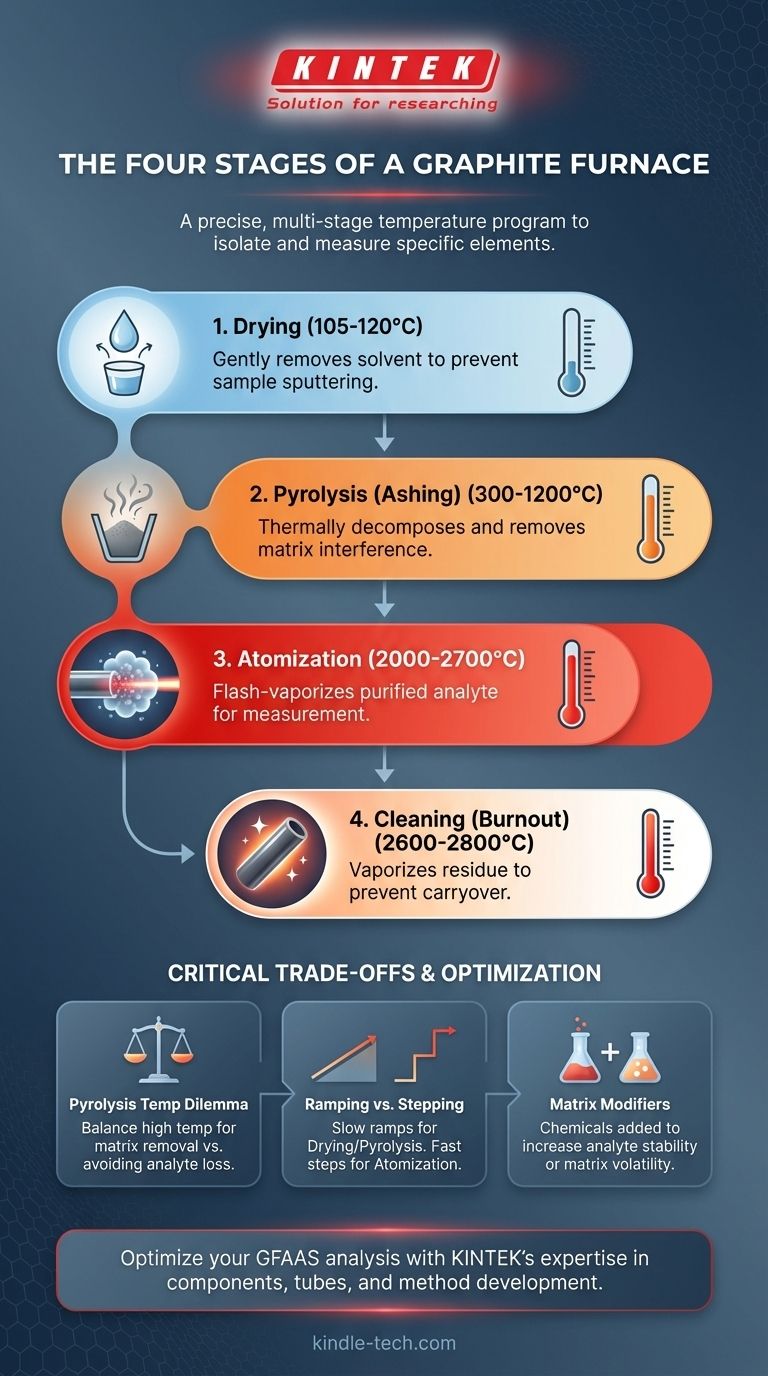
In analytical chemistry, a graphite furnace operates through a precise, multi-stage temperature program designed to isolate and measure a specific element. The four principal stages are drying, pyrolysis (or ashing), atomization, and cleaning. Each step systematically removes unwanted components of the sample, ensuring that the final measurement is accurate and free from interference.
The multi-stage temperature program is not just about heating; it's a systematic purification process. The goal is to carefully remove the sample matrix—solvents, salts, and organic matter—at lower temperatures so that only the target analyte remains for a clean measurement during the high-temperature atomization stage.
The Purpose of a Multi-Stage Temperature Program
A graphite furnace is a core component of a Graphite Furnace Atomic Absorption Spectrometer (GFAAS), an instrument capable of detecting elements at parts-per-billion concentrations.
The goal is to prepare a microscopic sample inside a graphite tube so that a beam of light can pass through a cloud of its vaporized atoms. The temperature program is the key to ensuring that the atom cloud being measured consists only of the element of interest, not the surrounding sample liquid or matrix.
A Stage-by-Stage Analysis
The furnace program is a series of timed temperature holds and ramps, each with a specific analytical purpose.
Stage 1: Drying
The first step is to gently remove the solvent (typically water or a dilute acid) from the sample droplet injected into the furnace.
This is usually done by slowly ramping the temperature to just above the solvent's boiling point, around 105-120°C. A slow ramp is critical to prevent the liquid from boiling explosively, which would sputter the sample and cause a significant loss of analyte.
Stage 2: Pyrolysis (Ashing)
This is arguably the most critical stage for complex samples. The goal of pyrolysis is to thermally decompose, or "ash," the sample matrix without losing the target analyte.
The temperature is raised significantly higher, often between 300°C and 1200°C. This process breaks down organic matter and vaporizes more volatile inorganic salts, which are then swept away by an internal inert gas flow (usually argon).
Stage 3: Atomization
This is the measurement stage. The furnace temperature is increased as rapidly as possible to a very high temperature, typically 2000-2700°C.
This sudden burst of energy flash-vaporizes the remaining, purified analyte, creating a dense, localized cloud of free, ground-state atoms inside the graphite tube. The instrument's light source passes through this cloud, and the amount of light absorbed is directly proportional to the concentration of the element.
Stage 4: Cleaning (Burnout)
After the measurement is complete, a final, maximum-temperature step is performed to ensure the furnace is ready for the next sample.
The temperature is raised to the furnace's limit, often 2600-2800°C, to vaporize any remaining residue. This "burnout" step prevents carryover, where analyte from a previous, more concentrated sample could artificially inflate the reading of the next one.
Understanding the Critical Trade-offs
Optimizing a furnace program requires balancing competing factors. Incorrect settings are a primary source of inaccurate results in GFAAS analysis.
The Pyrolysis Temperature Dilemma
The central challenge is setting the pyrolysis temperature. You want it to be as high as possible to remove the maximum amount of interfering matrix.
However, if the temperature is set too high, you risk prematurely vaporizing your target analyte along with the matrix. This leads to a lower signal during atomization and an erroneously low result. Finding the optimal pyrolysis temperature is the cornerstone of method development.
Ramping vs. Stepping
The speed of heating matters. The drying and pyrolysis stages often use a slow temperature ramp to allow for controlled, gentle removal of solvents and matrix components.
In contrast, the atomization stage requires a maximum-speed temperature step (a near-instant jump). This ensures all the analyte vaporizes at once, creating a sharp, narrow absorbance peak and providing the highest sensitivity.
The Role of Matrix Modifiers
For challenging samples, a chemical matrix modifier is often added. These are chemicals that either increase the thermal stability of the analyte (allowing for a higher pyrolysis temperature) or increase the volatility of the matrix (allowing it to be removed more easily). Common modifiers include palladium nitrate and magnesium nitrate.
Optimizing the Program for Your Analysis
The ideal temperature program depends entirely on your sample matrix and target analyte.
- If your primary focus is analyzing a simple, clean sample (e.g., dilute standard in water): You can use a more aggressive and faster temperature program, as matrix interference is minimal.
- If your primary focus is a complex matrix (e.g., seawater, blood, digested soil): A carefully optimized, slower program with a deliberate pyrolysis stage and potentially a matrix modifier is essential to achieve accuracy.
- If your primary focus is method development for a new analyte: You must create a pyrolysis curve by analyzing the sample at increasing pyrolysis temperatures to find the highest possible temperature before the analyte signal begins to drop.
A well-designed temperature program is the foundation of any successful graphite furnace analysis.

Summary Table:
| Stage | Purpose | Typical Temperature Range | Key Action |
|---|---|---|---|
| Drying | Remove solvent | 105-120°C | Gentle evaporation to prevent sputtering |
| Pyrolysis (Ashing) | Decompose sample matrix | 300-1200°C | Remove organic/inorganic interference |
| Atomization | Create atomic vapor cloud | 2000-2700°C | Flash-vaporize purified analyte for measurement |
| Cleaning | Remove residue | 2600-2800°C | Prevent carryover between samples |
Optimize your graphite furnace analysis with KINTEK's expertise!
Are you struggling with complex sample matrices or seeking to improve detection limits in your GFAAS work? KINTEK specializes in laboratory equipment and consumables that support precise temperature control and reliable graphite furnace operation. Our team can help you:
• Select the right furnace components for your specific analytical needs • Troubleshoot method development challenges, including pyrolysis temperature optimization • Provide high-quality graphite tubes and matrix modifiers for consistent performance
Contact our experts today to discuss how we can enhance your laboratory's analytical capabilities and ensure accurate, reproducible results for even the most challenging samples.
Get in touch with our technical team →
Visual Guide
Related Products
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Graphite Vacuum Furnace IGBT Experimental Graphitization Furnace
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
- Graphite Vacuum Continuous Graphitization Furnace
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
People Also Ask
- How does an alumina tube furnace with a controlled atmosphere simulate conditions in CSP environments? Master Accuracy.
- What is the function of alumina tubes and alumina wool in a pyrolysis furnace? Optimize Your Biochar Production Quality
- What is the pressure on a tube furnace? Essential Safety Limits for Your Lab
- What is the primary advantage of using a tube furnace? Achieve Superior Temperature and Atmosphere Control
- What are the advantages of using an alumina liner in a tube furnace for biomass combustion corrosion simulations?



















