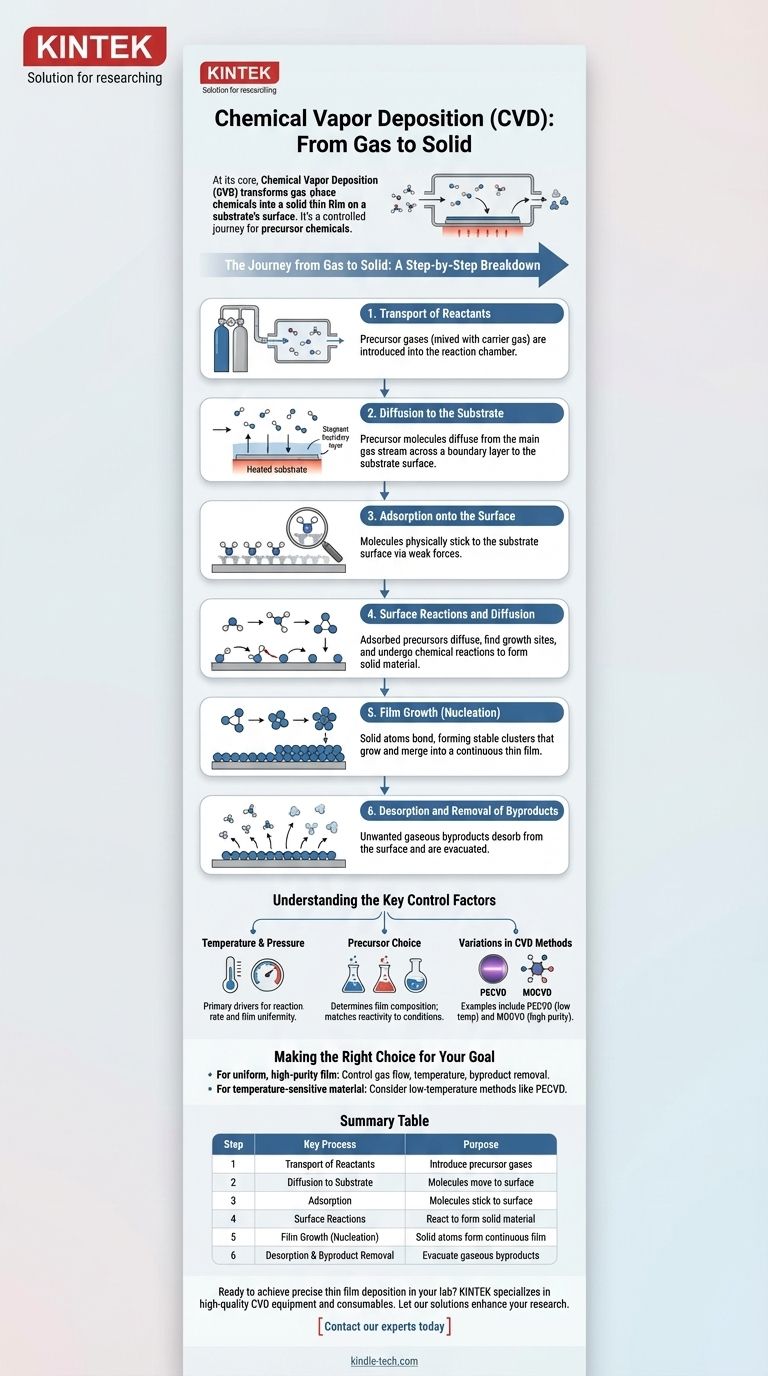
At its core, Chemical Vapor Deposition (CVD) is a sequence of events that transforms gas-phase chemicals into a solid thin film on a substrate's surface. The process begins with the transport of reactive gases into a chamber, followed by their diffusion to the target surface. Once there, the molecules adsorb, react, and form a stable film, while any gaseous byproducts are removed.
The entire CVD process can be understood as a controlled journey for precursor chemicals: they travel as a gas to a heated surface, undergo a chemical transformation into a solid, and deposit as a thin, uniform coating.

The Journey from Gas to Solid: A Step-by-Step Breakdown
The creation of a high-quality film via CVD depends on the precise execution of several sequential steps. Each stage plays a critical role in the final properties of the deposited material.
Step 1: Transport of Reactants
The process begins with the introduction of one or more volatile precursor gases into the reaction chamber.
These precursors, which contain the elements to be deposited, are often mixed with a carrier gas (like hydrogen or argon) to control their concentration and ensure smooth, stable delivery to the reaction zone.
Step 2: Diffusion to the Substrate
As the gas mixture flows over the heated substrate, a stagnant layer of gas, known as a boundary layer, forms just above the surface.
The precursor molecules must then move from the main gas stream across this boundary layer to physically reach the substrate surface. This transport is driven by a concentration gradient.
Step 3: Adsorption onto the Surface
Once a precursor molecule reaches the substrate, it must physically stick to the surface. This process is called adsorption.
The molecule is temporarily held on the surface by weak physical or chemical forces, making it available for the subsequent steps.
Step 4: Surface Reactions and Diffusion
This is the heart of the CVD process. The adsorbed precursor molecules, energized by the heated substrate, gain mobility and can diffuse across the surface.
They move to find energetically favorable growth sites, such as atomic steps or kinks. At these sites, the precursors undergo a chemical reaction—often breaking down (pyrolysis) or reacting with other precursors—to form the desired solid material.
Step 5: Film Growth (Nucleation)
The solid atoms produced by the surface reaction begin to bond together, forming stable clusters in a process called nucleation.
Over time, these initial nuclei grow and merge, eventually forming a continuous, thin film that builds up layer by layer on the substrate.
Step 6: Desorption and Removal of Byproducts
The chemical reactions that form the solid film almost always produce unwanted gaseous byproducts.
These byproduct molecules must desorb (detach) from the surface and be transported away from the substrate and out of the reaction chamber by the gas flow. Efficient removal is critical to prevent them from contaminating the growing film.
Understanding the Key Control Factors
The success of the CVD process hinges on precisely controlling the environment in which these steps occur. The interplay of temperature, pressure, and chemistry dictates the final outcome.
The Role of Temperature and Pressure
Temperature is the primary driver for the chemical reactions on the substrate surface. Higher temperatures generally increase the reaction rate but can also lead to unwanted gas-phase reactions.
Pressure, often a vacuum, is used to control the concentration of reactants and the thickness of the boundary layer, which directly influences the uniformity of the deposited film.
The Impact of Precursor Choice
The selection of precursor chemicals is fundamental, as it determines the composition of the final film. For example, depositing titanium carbide requires both a titanium-containing precursor and a carbon-containing precursor.
The chemical's volatility and reactivity must be matched to the process conditions.
Variations in CVD Methods
Different types of CVD exist to manipulate these steps. For instance, Plasma-Enhanced CVD (PECVD) uses a plasma to energize the gas, allowing reactions to occur at much lower temperatures.
Other methods, like Metalorganic CVD (MOCVD), use specific classes of precursors to achieve high-purity films for applications like manufacturing LEDs.
Making the Right Choice for Your Goal
Understanding this sequence allows you to troubleshoot issues and select the right parameters for a specific application.
- If your primary focus is creating a uniform, high-purity film: You must precisely control gas flow rates, maintain a stable substrate temperature, and ensure efficient removal of reaction byproducts.
- If your primary focus is depositing on a temperature-sensitive material: You should consider a low-temperature method like Plasma-Enhanced CVD (PECVD) to avoid damaging the substrate.
Ultimately, mastering CVD is about managing the journey of molecules from gas to a precisely engineered solid film.
Summary Table:
| Step | Key Process | Purpose |
|---|---|---|
| 1 | Transport of Reactants | Introduce precursor gases into the chamber |
| 2 | Diffusion to Substrate | Molecules move across the boundary layer to the surface |
| 3 | Adsorption | Molecules stick to the substrate surface |
| 4 | Surface Reactions | Precursors react to form solid material |
| 5 | Film Growth (Nucleation) | Solid atoms form a continuous thin film |
| 6 | Desorption & Byproduct Removal | Gaseous byproducts are evacuated from the chamber |
Ready to achieve precise thin film deposition in your lab? KINTEK specializes in high-quality CVD equipment and consumables, providing the reliable tools and expert support you need to master the gas-to-solid transformation. Let our solutions enhance your research and production outcomes. Contact our experts today to discuss your specific laboratory requirements!
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
People Also Ask
- What are the advantages of using a Low-Pressure Chemical Vapor Deposition (LPCVD) system? Master BN Nanocoatings on LATP
- What are the advantages of chemical Vapour deposition technique? Achieve Superior, Uniform Thin Films
- What core process conditions does a CVD furnace provide for graphene? Achieve High-Purity Crystalline Films
- How do Chemical Vapor Deposition (CVD) systems ensure material quality? Precision Control for Graphene-Coated Electrodes
- What is AC sputtering? A Guide to Deposition on Insulating Materials
- What is the pressure to create synthetic diamonds? HPHT vs. CVD Methods Explained
- What is the rate of physical vapor deposition? A Guide to Controlling Your Thin Film Growth
- What are the ingredients in synthetic diamonds? Unlocking the Science of Lab-Grown Carbon Crystals



















