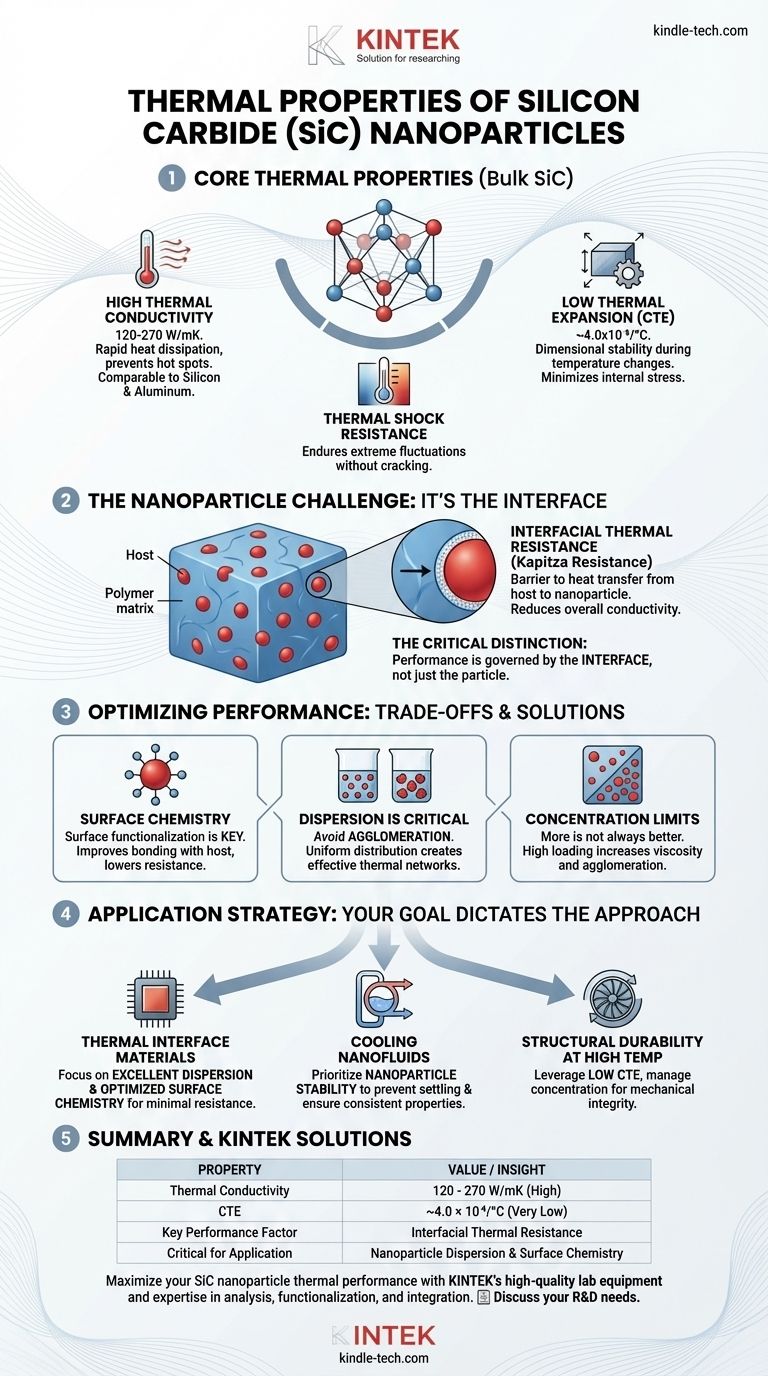
At its core, silicon carbide (SiC) is defined by two exceptional thermal characteristics: very high thermal conductivity and very low thermal expansion. Its thermal conductivity, ranging from 120-270 W/mK, allows it to dissipate heat rapidly, while its low coefficient of thermal expansion (4.0x10⁻⁶/°C) ensures it maintains dimensional stability during drastic temperature changes. This powerful combination is the reason SiC is a premier material for high-performance applications.
While the intrinsic properties of silicon carbide are outstanding, the performance of SiC nanoparticles within a system is not governed by the particle itself, but by the quality of the interface between the nanoparticle and its surrounding material.

The Core Thermal Properties of Silicon Carbide
Silicon carbide's fundamental properties make it uniquely suited for managing thermal loads and surviving thermal shock. Understanding these two attributes is the first step.
High Thermal Conductivity
The thermal conductivity of SiC (120-270 W/mK) is significantly higher than most other advanced ceramics and even some metals. For context, it is comparable to silicon (~150 W/mK) and aluminum (~235 W/mK).
This property means SiC can quickly pull heat away from a source and spread it throughout its volume, preventing the formation of damaging localized "hot spots."
Low Coefficient of Thermal Expansion (CTE)
SiC's CTE is remarkably low, meaning it expands and contracts very little when its temperature changes. This minimizes internal stress when a component is heated or cooled rapidly.
This stability is crucial in applications where materials are bonded together, as it prevents stress from building up at the joints due to mismatched expansion rates.
The Result: Superior Thermal Shock Resistance
When you combine high thermal conductivity with low thermal expansion, you get exceptional thermal shock resistance.
The material can endure rapid and extreme temperature fluctuations without cracking or failing. Heat is conducted away before it can create significant temperature gradients, and the small amount of expansion that does occur generates minimal internal stress.
The Critical Distinction: Bulk SiC vs. Nanoparticles
While the properties above describe the base material, the behavior changes when you use SiC in nanoparticle form, such as when creating a polymer composite or a nanofluid. The nanoparticle's interaction with its host material becomes the dominant factor.
The Impact of Interfacial Resistance
Heat does not flow seamlessly from a host material (like a polymer or oil) into a nanoparticle. This boundary creates a barrier to heat transfer known as interfacial thermal resistance (or Kapitza resistance).
This resistance acts as a bottleneck, meaning the overall thermal conductivity of the composite material will always be significantly lower than the pure SiC itself. The high conductivity of the nanoparticle is only useful if heat can get into it efficiently.
The Importance of Dispersion
To create an effective network for heat transfer, SiC nanoparticles must be distributed evenly throughout the host material.
However, nanoparticles have a strong tendency to agglomerate, or clump together. These clumps act like voids in the thermal network, drastically reducing the composite's ability to conduct heat and negating the benefit of adding the nanoparticles in the first place.
Understanding the Trade-offs
Simply adding SiC nanoparticles to a material does not guarantee improved thermal performance. You must account for several practical challenges.
Surface Chemistry is Key
The effectiveness of heat transfer across the particle-host boundary depends heavily on the chemical and physical bonding between the two.
Often, nanoparticles require surface functionalization—a process of chemically modifying their surface to improve compatibility with the host material. This modification can lower interfacial resistance and is critical for high-performance applications.
Concentration is Not a Silver Bullet
Increasing the concentration of SiC nanoparticles can improve thermal conductivity, but only up to a point.
At higher loading levels, the likelihood of agglomeration increases, and the mixture can become too viscous to process. Furthermore, adding too much filler can degrade other important properties of the host material, such as its flexibility or mechanical strength.
How to Apply This to Your Project
Your engineering strategy should be dictated by your primary goal. The "best" approach depends entirely on the application.
- If your primary focus is creating a thermal interface material or conductive composite: Concentrate on achieving excellent nanoparticle dispersion and optimizing surface chemistry to minimize interfacial resistance.
- If your primary focus is developing a cooling nanofluid: Prioritize nanoparticle stability in the fluid to prevent settling and agglomeration, ensuring the thermal properties remain consistent over time.
- If your primary focus is enhancing structural durability at high temperatures: Leverage SiC's low CTE to reduce thermal stress, but carefully manage nanoparticle concentration to avoid negatively impacting the mechanical integrity of the final part.
Understanding that the interface, not just the particle, governs performance is the key to successfully harnessing the power of silicon carbide nanoparticles.
Summary Table:
| Property | Value / Key Insight |
|---|---|
| Thermal Conductivity | 120 - 270 W/mK (High) |
| Coefficient of Thermal Expansion (CTE) | ~4.0 × 10⁻⁶/°C (Very Low) |
| Key Performance Factor | Interfacial Thermal Resistance |
| Critical for Application | Nanoparticle Dispersion & Surface Chemistry |
Ready to optimize your material's thermal performance with silicon carbide nanoparticles?
At KINTEK, we specialize in providing high-quality lab equipment and consumables to help you precisely analyze, functionalize, and integrate SiC nanoparticles into your polymers, composites, and nanofluids. Our expertise ensures you can overcome interfacial challenges and achieve superior heat dissipation and thermal stability.
Contact our experts today to discuss how KINTEK solutions can accelerate your R&D and enhance your product's thermal management capabilities.
Visual Guide
Related Products
- Silicon Carbide SiC Thermal Heating Elements for Electric Furnace
- Silicon Carbide (SIC) Ceramic Sheet Wear-Resistant Engineering Advanced Fine Ceramics
- Molybdenum Disilicide (MoSi2) Thermal Elements Electric Furnace Heating Element
- Hexagonal Boron Nitride HBN Ceramic Ring
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
People Also Ask
- What is the maximum temperature for silicon carbide heating element? The Real Limit for Your High-Temp Furnace
- What material is used for making heating element? Choose the Right Alloy for Your Application
- What are silicon carbide heating elements used for? Reliable High-Temp Heating for Industrial Processes
- What is silicon carbide rod heated to high temperature used as? A Premier Heating Element for Extreme Environments
- What is a silicon carbide heating element? Unlock Extreme Heat for Industrial Processes