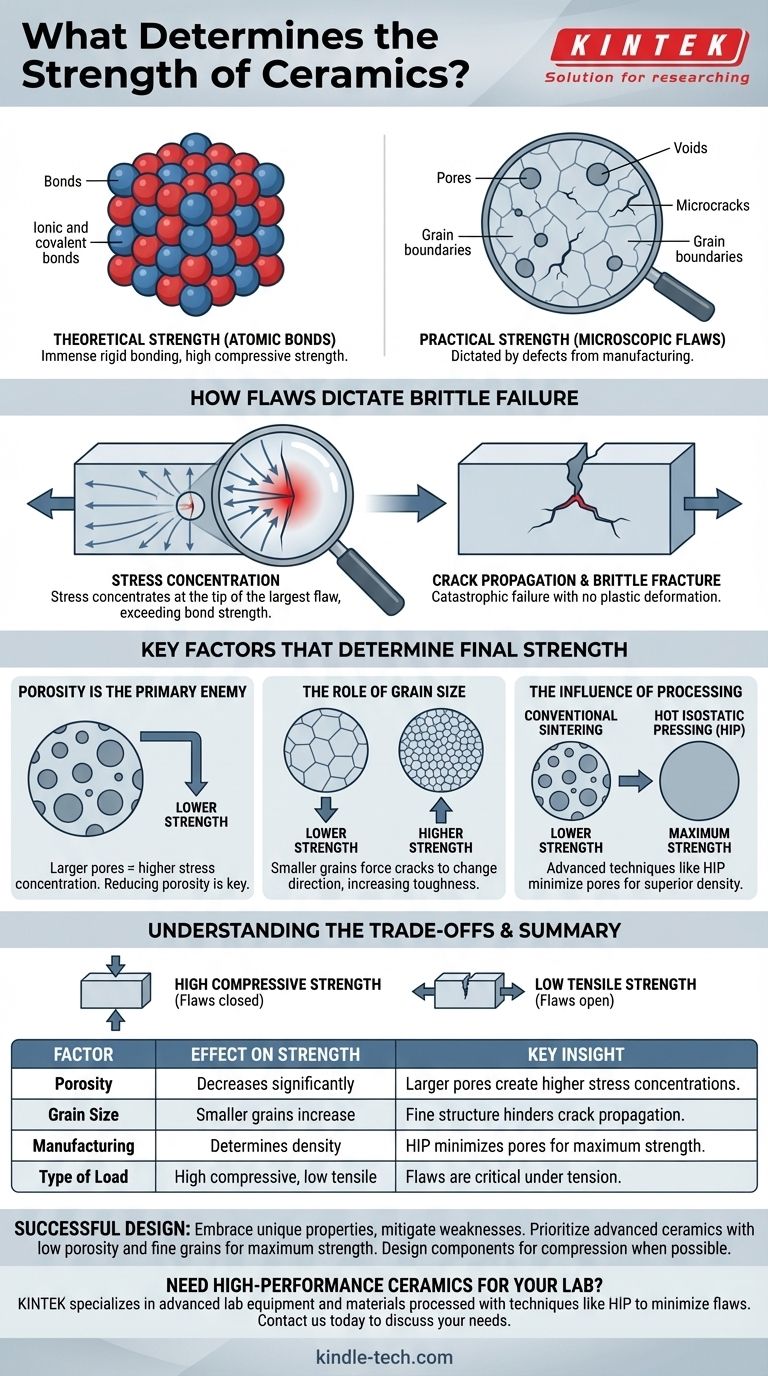
Ultimately, the strength of a ceramic is not determined by its powerful atomic bonds, but by the presence and size of its microscopic flaws. While ceramics possess immense theoretical strength due to their rigid ionic and covalent bonds, their practical, real-world strength is dictated by pre-existing defects like pores, microcracks, and grain boundaries that are introduced during manufacturing.
The core principle to understand is this: A ceramic's strength is a direct function of its imperfections. Stress concentrates at the tip of the largest flaw, and once that stress exceeds the material's intrinsic strength, a crack propagates catastrophically, leading to brittle failure.

The Paradox: Immense Strength Undermined by Flaws
Ceramics present a classic engineering paradox. Their internal structure is incredibly robust, yet they can fail under conditions that other materials, like metals, would easily withstand. This behavior is rooted in the conflict between their atomic bonding and their microstructure.
The Power of Atomic Bonds
Ceramics are characterized by extremely strong and rigid ionic and covalent bonds. These bonds lock atoms tightly in place, which is why ceramics are incredibly hard, resistant to high temperatures, and have very high compressive strength (resistance to being squeezed).
The Inevitable Reality of Flaws
However, no real-world ceramic is perfect. The process of manufacturing—mixing powders, pressing them into shape, and firing them at high temperatures (sintering)—inevitably creates microscopic defects. These include pores (tiny voids), microcracks, and inconsistent grain boundaries.
How Flaws Dictate Brittle Failure
These tiny, seemingly insignificant flaws are the true determinants of a ceramic's strength when it's pulled apart or bent. They act as initiation points for total failure.
Stress Concentration: The Breaking Point
When a ceramic part is put under tension (a pulling force), the stress is not distributed evenly. Instead, it concentrates intensely at the sharp tip of the largest, most severe flaw within the material.
This effect multiplies the applied force at that single point. A modest external load can generate a massive localized stress at a crack tip, easily exceeding the material's intrinsic bond strength.
Crack Propagation: The Point of No Return
In metals, this high stress would be relieved by plastic deformation—the material would bend and stretch. Ceramics cannot do this because their rigid bonds prevent atoms from sliding past one another.
Instead, the concentrated stress provides the energy to break the atomic bonds at the crack tip, causing the crack to grow. This process feeds itself, rapidly accelerating across the material until it fractures completely in a phenomenon known as brittle fracture.
Key Factors That Determine Final Strength
Understanding the mechanism of failure allows us to identify the critical factors that control the final, usable strength of a ceramic component.
Porosity Is the Primary Enemy
The single most important factor is porosity. Both the size and the quantity of pores directly impact strength. A larger pore creates a larger stress concentration site, making it the most likely point of failure. Reducing porosity is the most effective way to improve a ceramic's strength.
The Role of Grain Size
The strength of a ceramic can also be influenced by its grain size—the size of the individual crystalline regions within the material. Generally, a smaller and more uniform grain size increases strength and toughness. A crack propagating through the material is forced to change direction at each grain boundary, which consumes energy and makes fracture more difficult.
The Influence of Processing
The manufacturing method is what controls porosity and grain size. Advanced techniques like hot pressing or hot isostatic pressing (HIP) apply pressure during firing to squeeze out pores, resulting in a much denser and stronger final product compared to conventional sintering.
Understanding the Trade-offs
The inherent nature of ceramics creates a set of non-negotiable trade-offs that every engineer must consider.
High Compressive vs. Low Tensile Strength
The defining characteristic of ceramics is their immense strength under compression but relative weakness under tension. The flaws that initiate fracture under tension are simply pushed closed under compression, allowing the strong atomic bonds to bear the load.
The Absence of "Toughness"
Toughness is the ability of a material to absorb energy and deform before fracturing. Because ceramics lack a mechanism for plastic deformation, they have very low fracture toughness. This means failure is almost always sudden, catastrophic, and occurs with little to no warning.
Making the Right Choice for Your Goal
Your application's specific requirements will determine which ceramic properties matter most.
- If your primary focus is maximum mechanical strength: Prioritize advanced, technical ceramics with documented low porosity (<0.1%) and fine, controlled grain structures.
- If your primary focus is thermal stability or chemical resistance: You can often use more conventional ceramics, but you must design components to ensure they are loaded in compression, never in tension.
- If your primary focus is cost-effectiveness: Accept that traditional ceramics will have higher porosity and lower strength, and design a thicker or more robust geometry to compensate for the material's limitations.
Ultimately, successful design with ceramics comes from embracing their unique properties and mitigating their inherent weaknesses.
Summary Table:
| Factor | Effect on Strength | Key Insight |
|---|---|---|
| Porosity | Decreases strength significantly | Larger pores create higher stress concentrations, making failure more likely. |
| Grain Size | Smaller grains increase strength | A fine, uniform grain structure forces cracks to change direction, hindering propagation. |
| Manufacturing Process | Determines final density and flaw size | Hot Isostatic Pressing (HIP) minimizes pores for maximum strength. |
| Type of Load | High compressive, low tensile strength | Flaws are critical under tension but are closed under compression. |
Need high-performance ceramics for your lab? The strength and reliability of your ceramic components are directly tied to their manufacturing quality. At KINTEK, we specialize in advanced lab equipment and consumables, including materials processed with techniques like Hot Isostatic Pressing to minimize flaws and maximize performance. Let our experts help you select the right ceramic solution for your specific application—whether you need maximum mechanical strength, thermal stability, or chemical resistance.
Contact us today to discuss how we can support your laboratory's needs with precision-engineered ceramics.
Visual Guide
Related Products
- Custom-Made Alumina Zirconia Special-Shaped Ceramic Plates for Engineering Advanced Fine Ceramics Processing
- Precision Machined Zirconia Ceramic Ball for Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Alumina Al2O3 Ceramic Rod Insulated for Industrial Applications
- Zirconia Ceramic Gasket Insulating Engineering Advanced Fine Ceramics
- Engineering Advanced Fine Ceramics Head Tweezers with Pointed Elbow Zirconia Ceramic Tip
People Also Ask
- What is the material used in high temperature furnace? Selecting the Right Ceramic for Extreme Heat
- Why ceramics can withstand high temperature? Unlock the Secrets of Atomic Structure
- What are the advantages of dental ceramics? Achieve a Natural, Durable Smile
- What is another name for dental ceramic? Discover the Porcelain & Modern Material Options
- How is the poor thermal-shock resistance of pure alumina typically mitigated? Improve Durability with Alumino-Silicates
- What is the maximum operating temperature of alumina? The Critical Role of Purity and Form
- What temperature is alumina fired at? Unlock the Key to Perfect Ceramic Sintering
- What material is used for pusher plates? Discover Mullite's Superior Chemical and Thermal Shock Resistance