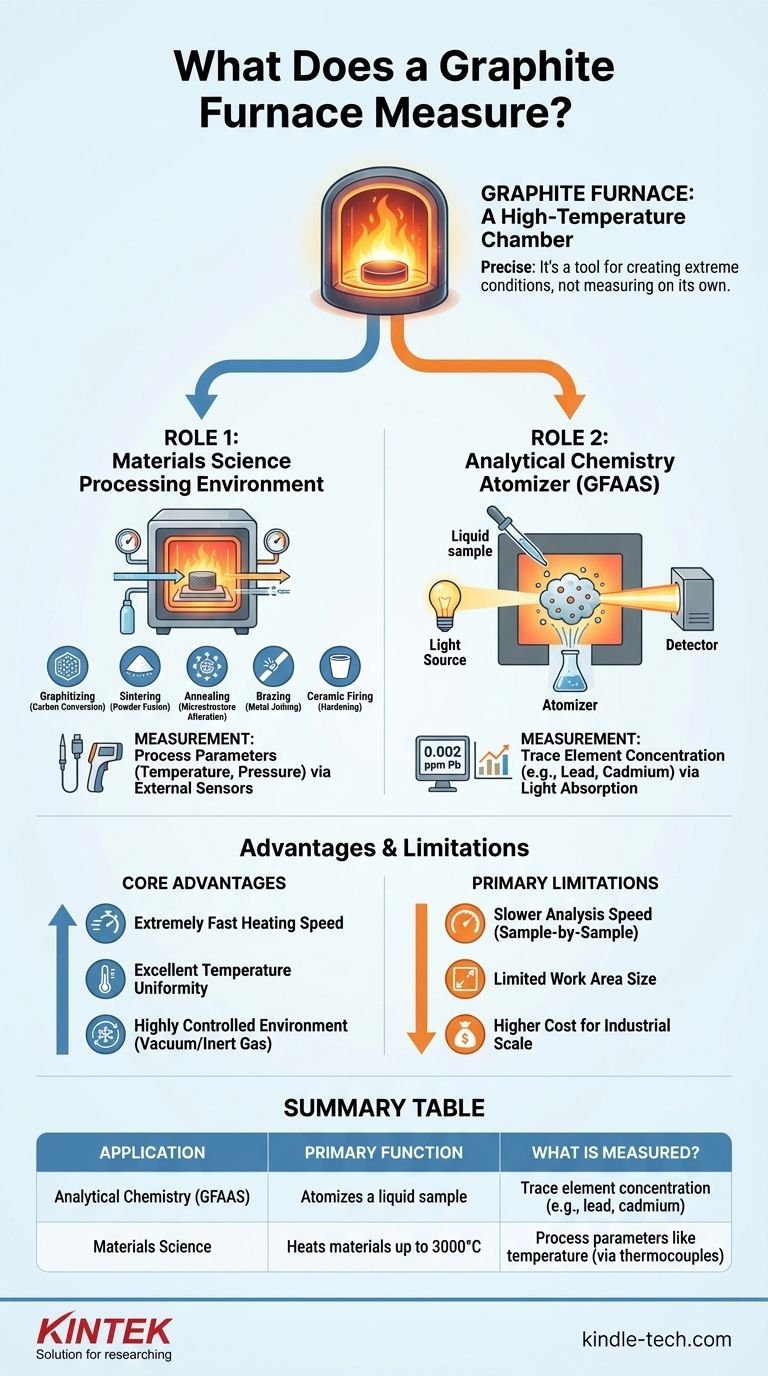
To be precise, a graphite furnace does not inherently measure anything on its own. Instead, it is a sophisticated high-temperature chamber that creates extreme conditions necessary for two distinct purposes: processing advanced materials or, more commonly, preparing a sample to measure the concentration of trace elements as part of a larger analytical system.
The core function of a graphite furnace is to provide a precisely controlled, ultra-high temperature environment. This environment is used either to physically alter materials or to vaporize a sample for chemical analysis via atomic absorption spectroscopy.

The Two Primary Roles of a Graphite Furnace
The term "graphite furnace" can be confusing because it refers to a core component used in two very different fields: materials science and analytical chemistry. Understanding your application is key to understanding its function.
Role 1: A High-Temperature Processing Environment
In materials science and industrial manufacturing, a graphite furnace is essentially an advanced oven. Its purpose is to heat materials to temperatures as high as 3000°C in a highly controlled atmosphere.
This process is used for applications like:
- Graphitizing: Converting carbon-based materials into a more ordered, graphitic structure.
- Sintering: Fusing powders together to form a solid mass without melting them.
- Annealing: Altering the microstructure of a material to improve its ductility and reduce hardness.
- Brazing: Joining two or more metal items by melting and flowing a filler metal into the joint.
- Ceramic Firing: Hardening ceramic materials at extreme temperatures.
In this context, the furnace itself doesn't measure a property of the material. Instead, internal sensors like thermocouples or pyrometers are used to measure and control the furnace's temperature during the process.
Role 2: The Atomizer in Analytical Chemistry (GFAAS)
This is the most common technical meaning of a graphite furnace. In this role, it is a critical component of an instrument called a Graphite Furnace Atomic Absorption Spectrometer (GFAAS).
The furnace's job is to take a tiny liquid sample and, through a rapid heating program, vaporize and then atomize it. This converts the elements within the sample into a cloud of free, neutral atoms.
Light from a specific lamp is then passed through this atomic cloud. The atoms of the target element (e.g., lead, cadmium) will absorb this light, and a detector measures the amount of light absorbed.
This absorption is directly proportional to the concentration of the element in the original sample. Therefore, in this context, the graphite furnace is the key that enables the measurement of trace and ultra-trace element concentrations, often at parts-per-billion levels.
Understanding the Key Advantages and Limitations
A graphite furnace is chosen for its unique capabilities, but it's important to recognize its operational trade-offs.
The Core Advantages
The design offers several key benefits. It provides extremely fast heating speeds and excellent temperature uniformity within the graphite tube.
Furthermore, it allows for a highly controlled environment. Processes can be run under a vacuum or backfilled with an inert gas (like argon) to prevent unwanted chemical reactions, such as oxidation.
The Primary Limitations
In analytical chemistry (GFAAS), the main limitation is speed. The process analyzes samples one at a time with heating and cooling cycles, making it much slower than techniques that can analyze a continuous stream of sample.
In materials science, the primary constraints are often size and cost. The usable work area can be small (e.g., a few inches in diameter), making it unsuitable for large-scale production compared to other types of industrial furnaces.
Making the Right Choice for Your Goal
Ultimately, how you use a graphite furnace depends entirely on your objective.
- If your primary focus is materials processing: You are using the furnace as a powerful tool to create or modify materials under extreme, controlled thermal conditions.
- If your primary focus is chemical analysis: You are using the furnace as part of a GFAAS system to achieve exceptional sensitivity for measuring trace element concentrations in a sample.
Understanding these two distinct applications is the key to mastering the purpose and power of the graphite furnace.
Summary Table:
| Application | Primary Function | What is Measured? |
|---|---|---|
| Analytical Chemistry (GFAAS) | Atomizes a liquid sample | Trace element concentration (e.g., lead, cadmium) |
| Materials Science | Heats materials up to 3000°C | Process parameters like temperature (via thermocouples) |
Need precise trace element analysis or advanced high-temperature processing? KINTEK specializes in high-performance graphite furnaces and lab equipment. Our solutions deliver the precise temperature control and sensitivity your laboratory requires for accurate results. Contact our experts today to find the perfect system for your application!
Visual Guide
Related Products
- Graphite Vacuum Furnace High Thermal Conductivity Film Graphitization Furnace
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
- Graphite Vacuum Furnace IGBT Experimental Graphitization Furnace
- Graphite Vacuum Continuous Graphitization Furnace
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
People Also Ask
- What is the application of graphite furnace? Essential for High-Temp Material Processing & Synthesis
- What is the graphite furnace technique? Achieve Extreme Temperatures for Advanced Materials
- What is the disadvantage of graphite furnace? Managing Reactivity and Contamination Risks
- What is the graphite furnace method? Achieve Ultra-High Temperatures with Purity & Speed
- What happens to graphite when heated? Unlock Its High-Temperature Potential or Risk Oxidation
- Why is the graphite furnace technique more sensitive than flame based vaporization methods for atomic absorption? Unlock Superior Trace Analysis
- What is the purpose of a graphite furnace? Achieve Extreme Temperatures for Advanced Materials
- What are the advantages of graphite? Unlock Superior Performance in High-Temperature Processes



















