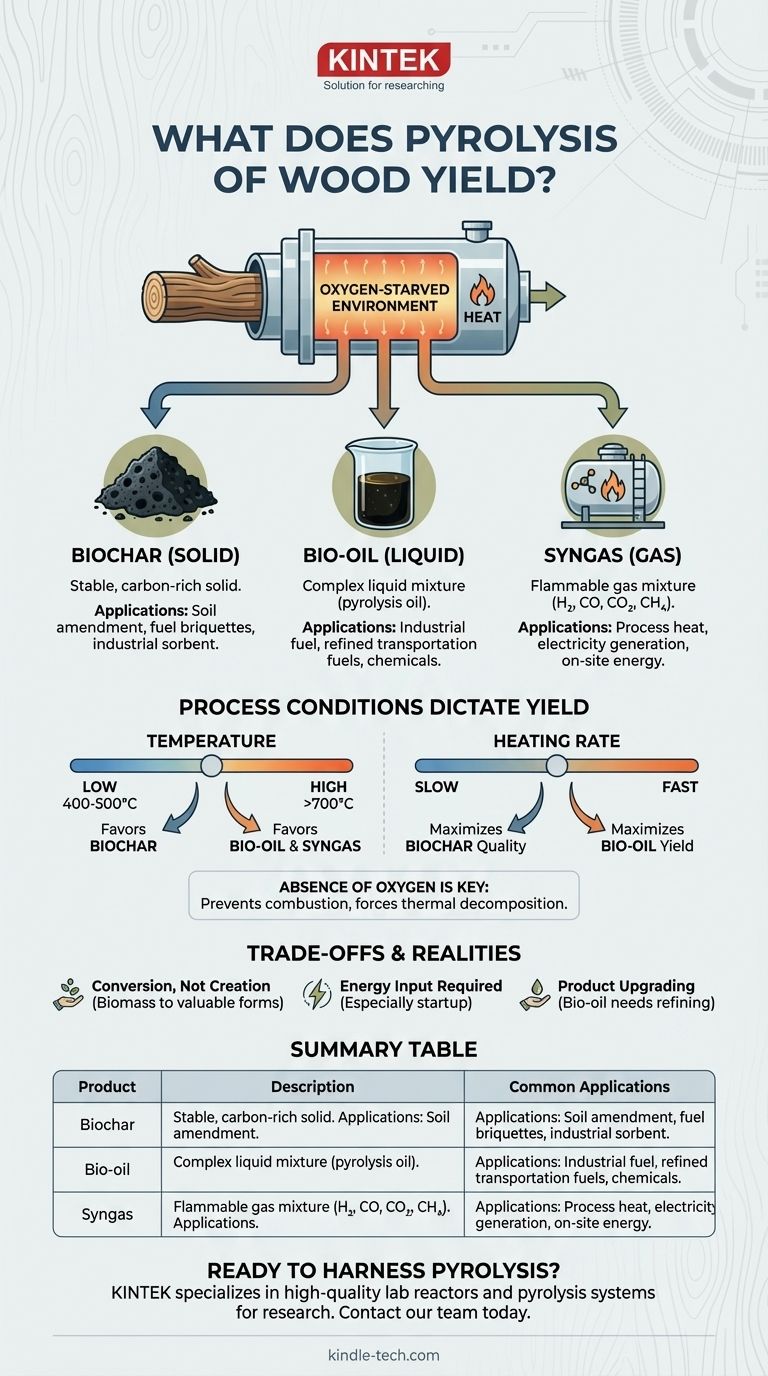
In short, the pyrolysis of wood yields three distinct products. In a high-temperature, oxygen-starved environment, wood doesn't burn; it decomposes into a solid material called biochar, a liquid known as bio-oil (or pyrolysis oil), and a combustible gas mixture called syngas. The specific proportion of each product is not fixed but is deliberately controlled by the process conditions.
Pyrolysis is best understood not as a single outcome, but as a versatile thermal conversion platform. By precisely controlling factors like temperature and heating rate, you can intentionally shift the process to favor the production of either solid char, liquid fuel, or flammable gas, depending on your primary goal.

The Three Core Products of Wood Pyrolysis
Pyrolysis breaks down the complex organic matter in wood into simpler, more valuable components. Each of the three resulting products has its own distinct characteristics and applications.
The Solid Product: Biochar
Biochar is the stable, carbon-rich solid that remains after the volatile components of the wood have been driven off. It is essentially a form of charcoal.
Its highly porous structure makes it extremely useful as a soil amendment, where it can improve water retention, nutrient availability, and house beneficial microbes. It is also used to create fuel briquettes or as an industrial sorbent.
The Liquid Product: Bio-oil
Bio-oil, also known as pyrolysis oil, is a complex liquid mixture of water, tars, and hundreds of different organic compounds, including wood vinegar.
This dense, dark liquid can be used directly as an industrial fuel or, more commonly, can be refined and upgraded into more valuable transportation fuels, similar to biodiesel. It is also a potential source for specialty chemicals.
The Gaseous Product: Syngas
Syngas is the collection of non-condensable gases released during pyrolysis, including hydrogen, carbon monoxide, carbon dioxide, and methane.
This gas mixture is flammable and has a significant energy value. In most pyrolysis operations, the syngas is captured and recycled to provide the heat needed to sustain the reaction, making the process more energy-efficient. It can also be used to generate electricity and heat.
How Process Conditions Dictate the Yield
The key to understanding pyrolysis is knowing that you can steer the outcome. The final yields of char, oil, and gas are a direct result of the specific process parameters used.
The Critical Role of Temperature
Temperature is the most influential factor. There is a clear and direct relationship between heat and the final product distribution.
A lower temperature range (400–500 °C) slows the decomposition process, favoring the creation of a solid structure. This is ideal for maximizing biochar production.
Conversely, higher temperatures (above 700 °C) cause the wood's structure to break down much more rapidly and completely, maximizing the yield of bio-oil and syngas.
The Impact of Heating Rate
The speed at which the wood is heated also plays a crucial role.
Slow pyrolysis, where the wood is heated gradually over a longer period, allows more carbon to remain in the solid residue. This method is specifically employed to produce the highest quantity and quality of biochar.
The Absence of Oxygen
This is the defining condition of pyrolysis. Heating wood in the presence of oxygen results in combustion, where the material burns away, leaving only a small amount of mineral ash.
By excluding oxygen, the wood is forced to thermally decompose rather than burn, preserving the carbon in the form of char, oil, and gas.
Understanding the Trade-offs and Realities
While a powerful technology, pyrolysis is governed by practical constraints and chemical realities that are important to recognize.
It's a Conversion, Not Creation
Pyrolysis does not create energy or material from nothing. It simply converts low-density, often low-value, biomass into denser, more valuable, and more versatile forms of fuel and material.
Energy Input is Required
The process is energy-intensive, especially during startup. While it can become partially self-sustaining by burning its own syngas product, an external energy source is required to bring the reactor up to the necessary operating temperature.
Product Upgrading Adds Complexity
The direct outputs of pyrolysis, particularly bio-oil, are not finished products. Bio-oil is typically acidic, unstable, and requires significant refining and upgrading before it can be used as a modern transportation fuel, adding cost and complexity to the overall process.
Making the Right Choice for Your Goal
The optimal pyrolysis strategy depends entirely on the desired end product. You must align the process parameters with your primary objective.
- If your primary focus is soil improvement and carbon sequestration: Slow, lower-temperature pyrolysis is the optimal path to maximize high-quality biochar production.
- If your primary focus is producing liquid biofuels: Fast pyrolysis at higher temperatures is necessary to crack the biomass into the highest possible yield of bio-oil.
- If your primary focus is on-site energy generation: A balanced process that uses the resulting syngas to power the operation or an electrical generator is the most efficient approach.
Ultimately, pyrolysis offers a flexible and powerful method for transforming wood from a simple raw material into a spectrum of valuable commodities.
Summary Table:
| Product | Description | Common Applications |
|---|---|---|
| Biochar (Solid) | Stable, carbon-rich solid residue | Soil amendment, fuel briquettes, industrial sorbent |
| Bio-oil (Liquid) | Complex liquid mixture of organic compounds | Industrial fuel, refined transportation fuels, chemicals |
| Syngas (Gas) | Flammable mixture of hydrogen, CO, CO₂, methane | Process heat, electricity generation, on-site energy |
Ready to harness the power of pyrolysis for your biomass conversion needs? The right laboratory equipment is crucial for process development and optimization. KINTEK specializes in high-quality lab reactors and pyrolysis systems designed for research and pilot-scale production. Whether your goal is biochar for soil enhancement, bio-oil for fuel, or syngas for energy, our experts can help you select the right tools to achieve precise temperature control and optimal yields.
Contact our team today to discuss how KINTEK's laboratory solutions can advance your pyrolysis projects.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace for Activated Carbon Regeneration
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Laboratory Vacuum Tilt Rotary Tube Furnace Rotating Tube Furnace
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What temperature is needed for porcelain? A Guide to Cone 6 and Cone 10 Firing
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- How to regenerate activated carbon? Master the 3-Stage Thermal Process for Cost Savings
- What is the temperature of a rotary hearth furnace? Find the Right Heat for Your Process
- What temperature is a carbon regeneration kiln? Master the 650°C-800°C Range for Optimal Results



















