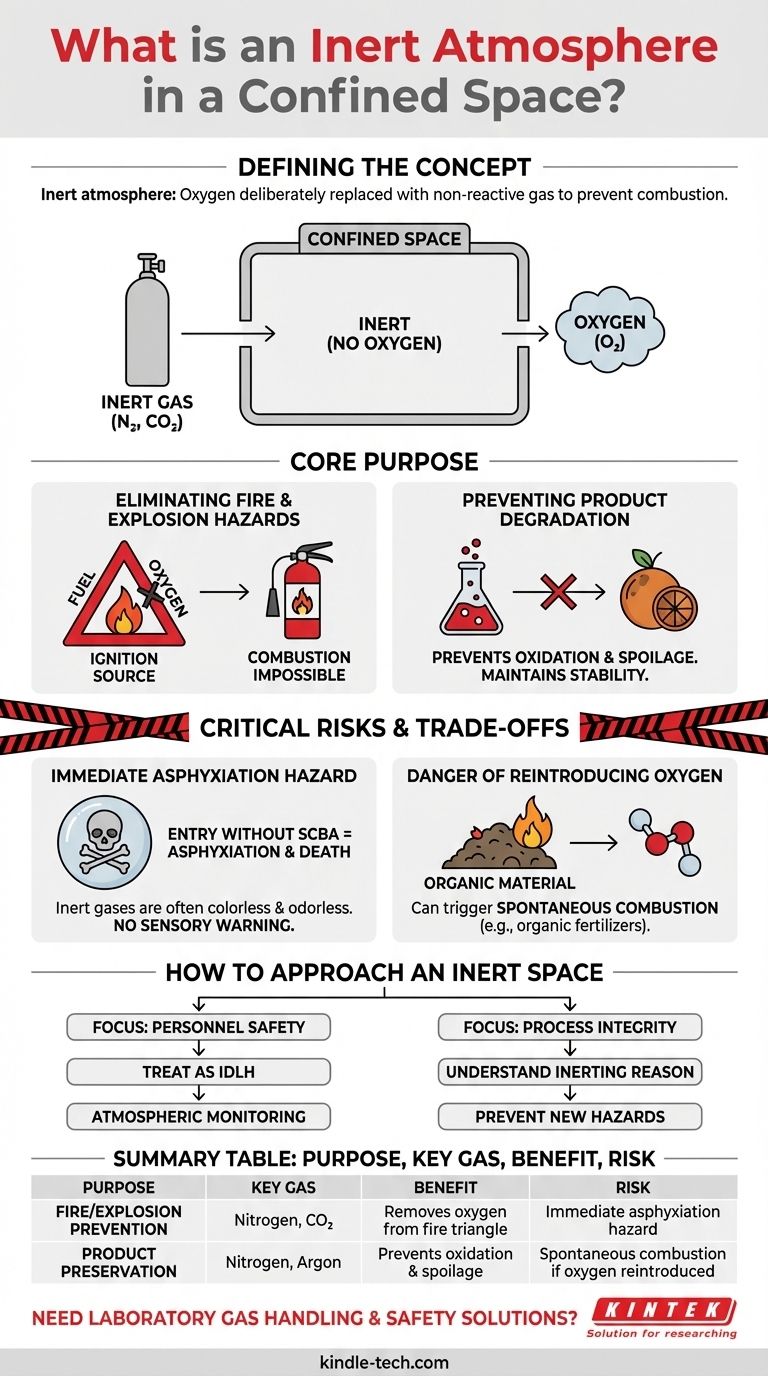
In a confined space, an inert atmosphere is one where the oxygen has been deliberately replaced with a non-reactive gas, making the environment incapable of supporting combustion. This is typically achieved by purging the space with gases like nitrogen or carbon dioxide to prevent fires or stop a product from spoiling due to oxidation.
An inert atmosphere is a critical engineering control that eliminates fire hazards and prevents product degradation by removing oxygen. However, this process creates an environment that is immediately lethal to humans and introduces new risks if the atmosphere is not managed with extreme caution.

The Core Purpose of Inerting a Space
Understanding why a space has been made inert is the first step in managing its risks. The two primary drivers are the prevention of combustion and the preservation of materials.
Eliminating Fire and Explosion Hazards
The most common reason for inerting is to prevent a fire or explosion. For a fire to occur, three elements are needed: fuel, an ignition source, and oxygen.
By purging a confined space with an inert gas, you remove the oxygen from this "fire triangle," making combustion impossible even if a fuel and ignition source are present.
Preventing Product Degradation
Many materials react with oxygen in a process called oxidation. This can degrade the quality of a product, cause spoilage, or create unwanted chemical reactions.
For example, certain organic products or chemicals must be stored in an inert atmosphere to maintain their stability and prevent them from spoiling or breaking down over time.
Critical Risks and Inherent Trade-offs
While inerting solves specific operational problems, it creates an environment with its own set of severe hazards that must be managed.
The Immediate Asphyxiation Hazard
An inert atmosphere is incompatible with human life. Because the oxygen has been displaced, entering an inert space without a self-contained breathing apparatus would lead to asphyxiation and death within minutes.
Inert gases like nitrogen and carbon dioxide are often colorless and odorless, providing no sensory warning of the danger.
The Danger of Reintroducing Oxygen
It is critical to know why a space was inerted before returning it to a breathable atmosphere. Certain products, like organic fertilizers, can undergo self-heating.
Reintroducing oxygen to such a product can trigger spontaneous combustion, creating the very fire hazard the inerting process was designed to prevent. This highlights the need to understand the material inside the space before altering the atmosphere.
How to Approach an Inert Confined Space
Your operational goal dictates your primary safety considerations when dealing with an inert atmosphere.
- If your primary focus is personnel safety during entry: You must treat any inerted space as immediately dangerous to life and health (IDLH) until proven otherwise with calibrated atmospheric monitoring equipment.
- If your primary focus is process integrity: You must understand the specific reason for the inerting before ever reintroducing oxygen to avoid catastrophic product loss or creating a new fire hazard.
Ultimately, managing an inert atmosphere is a fundamental exercise in risk assessment and control.
Summary Table:
| Purpose | Key Gas Used | Primary Benefit | Primary Risk |
|---|---|---|---|
| Fire/Explosion Prevention | Nitrogen, CO₂ | Removes oxygen from the fire triangle | Immediate asphyxiation hazard |
| Product Preservation | Nitrogen, Argon | Prevents oxidation and spoilage | Spontaneous combustion if oxygen is reintroduced |
Need to create or manage a safe inert atmosphere in your lab? KINTEK specializes in laboratory equipment and consumables, including gas handling and safety solutions. Our expertise ensures your processes are both effective and safe. Contact our safety experts today to discuss your specific needs and protect your personnel and products.
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- How we can develop inert atmosphere for a chemical reaction? Master Precise Atmospheric Control for Your Lab
- Why is high-precision chemical activation equipment necessary for biomass-derived activated carbon? Top Quality Guide
- What are the different types of brazing gas? Choose the Right Atmosphere for Strong, Clean Joints
- What are the typical air-to-gas ratios for endothermic generators? Optimize Natural Gas and Propane Settings
- What is the main hazard associated with the use of inert gases? The Silent Danger of Oxygen Displacement
- What is the function of a high-temperature atmosphere furnace in the carbonization of cellulose waste? Expert Guide
- Why Use an Atmosphere Protection Furnace with Argon for FM Steel? Ensure Integrity and Prevent Oxidation
- What kind of environment does an atmosphere tube furnace provide for Ti2AlN? Achieve Pure Ceramic Sintering Results



















