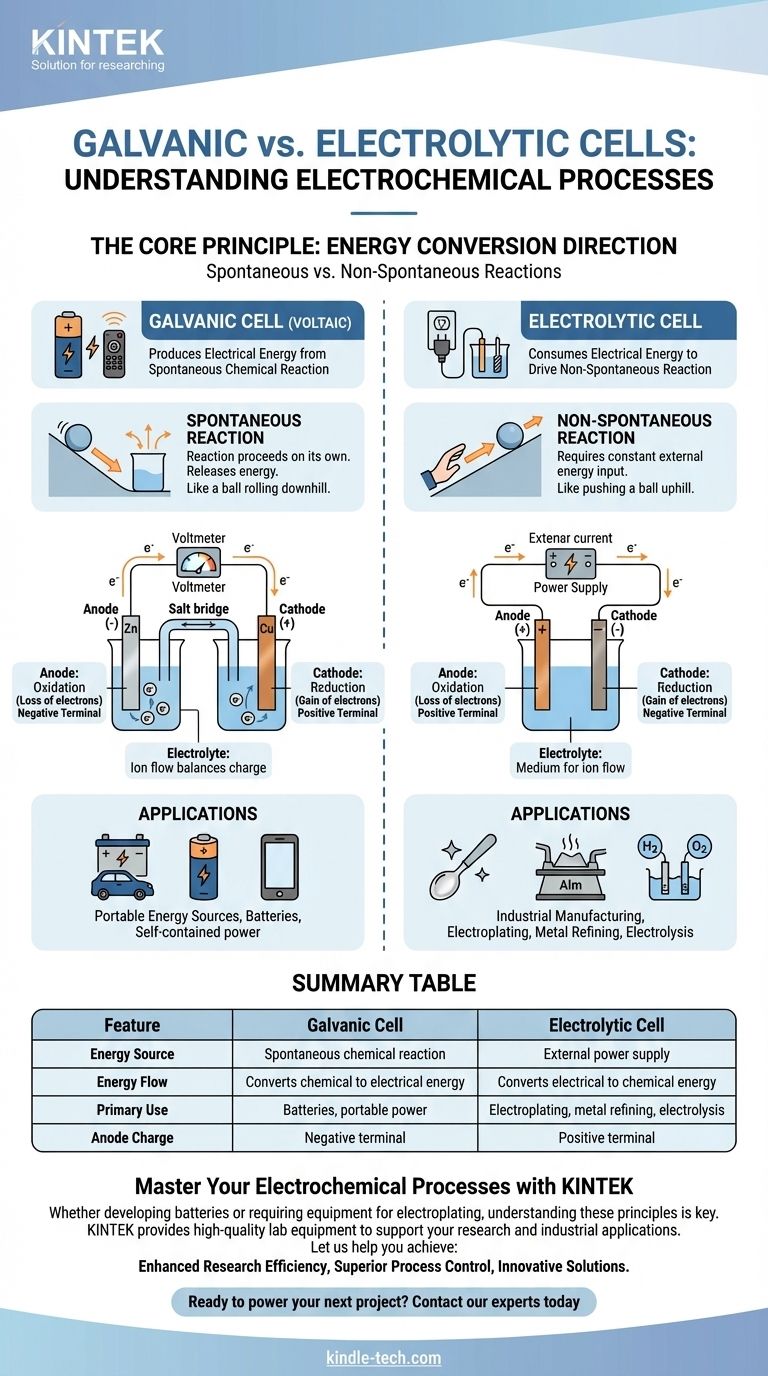
At its core, a galvanic cell is an electrochemical cell that produces electrical energy from a spontaneous chemical reaction, like a battery powering your remote. In contrast, an electrolytic cell consumes electrical energy from an external source to force a non-spontaneous chemical reaction to occur, a process used in metal plating and purification.
The fundamental distinction is the direction of energy conversion. A galvanic cell converts stored chemical energy into electrical energy, while an electrolytic cell converts external electrical energy into chemical energy.

The Core Principle: Spontaneous vs. Non-Spontaneous Reactions
The behavior of any electrochemical cell is dictated by the natural tendency of its chemical reaction. This tendency is the key to understanding why one cell produces power and the other requires it.
How Galvanic Cells Generate Power
A galvanic cell, also known as a voltaic cell, is built around a spontaneous chemical reaction.
This is a reaction that proceeds on its own without external intervention, releasing energy in the process. Think of it as a ball rolling downhill.
This release of energy pushes electrons through an external circuit, creating an electric current. This is the principle behind all non-rechargeable and discharging rechargeable batteries.
How Electrolytic Cells Consume Power
An electrolytic cell drives a non-spontaneous chemical reaction.
This is a reaction that will not happen on its own and requires a constant input of energy to proceed. It is the equivalent of pushing a ball uphill.
An external power source, like a power supply, provides the necessary voltage to force electrons to flow against their natural direction, driving the desired chemical change.
A Practical Look at Cell Function
While their purposes are opposite, both cells share common components that operate under the same fundamental rules of chemistry, but with a critical difference in polarity.
The Anode and Cathode: A Tale of Two Polarities
In both cell types, the anode is always where oxidation occurs (loss of electrons) and the cathode is where reduction occurs (gain of electrons).
However, their electrical charge is reversed. In a galvanic cell, the anode is the negative terminal because the spontaneous reaction releases electrons from it.
In an electrolytic cell, the anode is the positive terminal because the external power source pulls electrons away from it, forcing oxidation to happen.
The Role of the Electrolyte
Both cells contain an electrolyte, typically a solution containing ions.
This medium is essential for completing the electrical circuit. While electrons flow through the external wire, ions flow through the electrolyte to balance the charge at the electrodes.
Understanding the Applications and Trade-offs
The opposing functions of these cells lead to vastly different real-world applications and inherent limitations.
Galvanic Cells: Portable Energy Sources
The primary use for galvanic cells is to act as batteries. They provide a portable, self-contained source of electrical power.
The trade-off is that the chemical reactants within the cell are finite. Once they are consumed, the cell stops producing power and must be either discarded or recharged.
Electrolytic Cells: Industrial Manufacturing and Purification
Electrolytic cells are workhorses of industry, used for processes that are otherwise chemically impossible or inefficient.
Key applications include electroplating (coating one metal with another), refining metals like aluminum and copper, and the electrolysis of water to produce hydrogen and oxygen. Their main limitation is the requirement for a significant and continuous supply of external electrical energy.
How to Apply This Knowledge
Your choice between these concepts depends entirely on whether your goal is to generate power or to induce a chemical change.
- If your primary focus is generating electricity from a chemical reaction: You are working with the principles of a galvanic cell, such as when designing or understanding a battery.
- If your primary focus is using electricity to create a product or refine a substance: You are working with the principles of an electrolytic cell, common in chemical manufacturing and metallurgy.
Understanding this division is the first step toward mastering the practical application of electrochemistry.
Summary Table:
| Feature | Galvanic Cell | Electrolytic Cell |
|---|---|---|
| Energy Source | Spontaneous chemical reaction | External power supply |
| Energy Flow | Converts chemical to electrical energy | Converts electrical to chemical energy |
| Primary Use | Batteries, portable power | Electroplating, metal refining, electrolysis |
| Anode Charge | Negative terminal | Positive terminal |
Master Your Electrochemical Processes with KINTEK
Whether you are developing new battery technologies or require precise equipment for electroplating and metal purification, understanding these core electrochemical principles is just the beginning. KINTEK specializes in providing high-quality lab equipment and consumables to support your research and industrial applications.
Let us help you achieve:
- Enhanced Research Efficiency: With reliable equipment for testing and developing both galvanic and electrolytic systems.
- Superior Process Control: For consistent results in electroplating, electrolysis, and material synthesis.
- Innovative Solutions: Tailored to the unique needs of laboratories and production facilities.
Ready to power your next project? Contact our experts today to find the perfect solution for your laboratory needs!
Visual Guide
Related Products
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell for Coating Evaluation
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- H Type Electrolytic Cell Triple Electrochemical Cell
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
People Also Ask
- What is the structure of an H-type exchangeable membrane electrolytic cell? A Guide to Precise Electrochemical Separation
- What is the purpose of the double-layer structure in the H-type electrolytic cell? Achieve Precise Thermal Control
- What are the standard opening specifications for an H-type exchangeable membrane electrolytic cell? Asymmetrical Ports for Precise Electrochemistry
- What is the typical experimental system used with a double-layer water-bath electrolytic cell? Achieve Precise Electrochemical Control
- What are the typical volumes and aperture configurations for a double-layer water-bath electrolytic cell? Optimize Your Electrochemical Setup



















