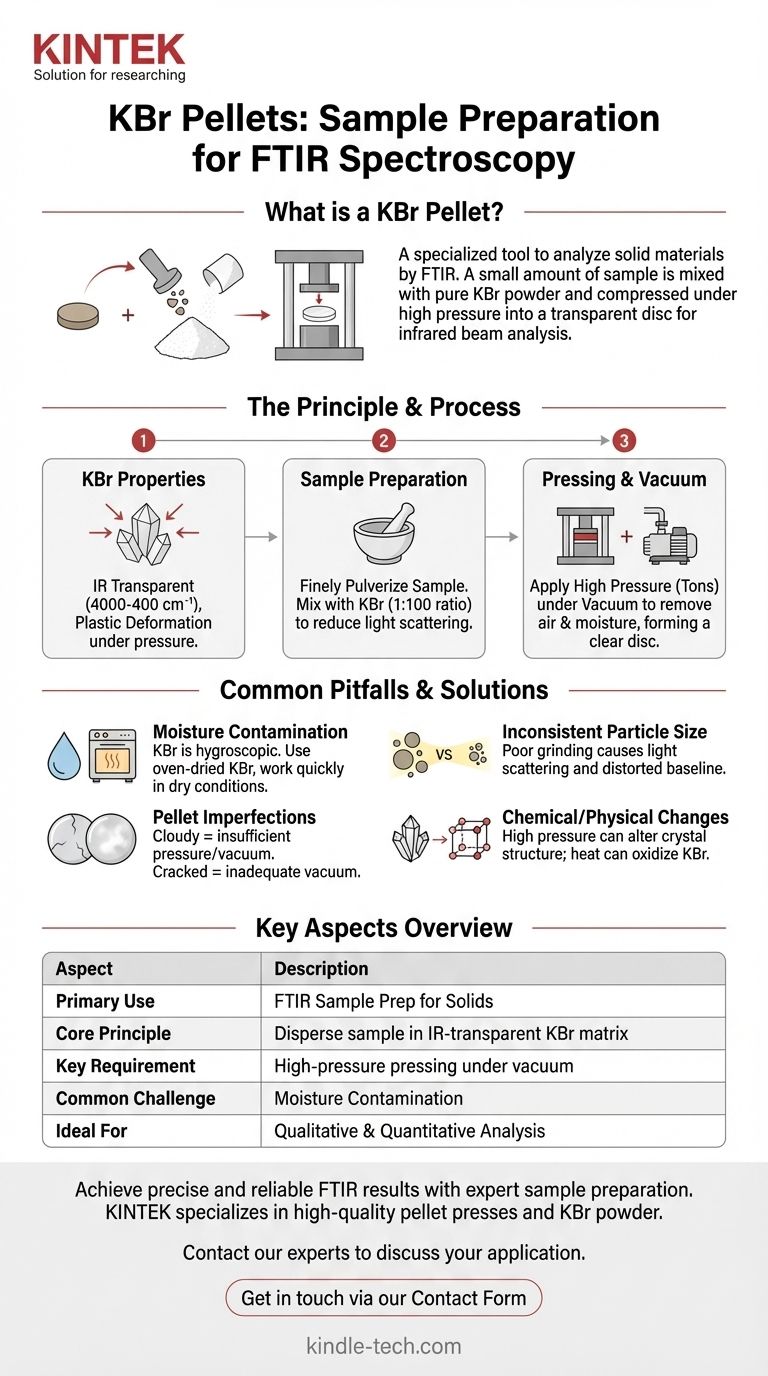
At its core, a KBr pellet is a specialized sample preparation tool used for analyzing solid materials with Fourier Transform Infrared (FTIR) Spectroscopy. The technique involves mixing a small amount of a solid sample with pure potassium bromide (KBr) powder and compressing the mixture under high pressure to form a small, transparent disc. This disc can then be placed directly in the path of an infrared beam for analysis.
The fundamental challenge in infrared spectroscopy is that solid samples are typically opaque to IR radiation. The KBr pellet method solves this by dispersing the sample within an IR-transparent matrix (the KBr), effectively turning an opaque solid into a measurable, semi-transparent window.

The Principle Behind the KBr Pellet Method
To understand why this technique is so widely used, it's essential to grasp the properties of KBr and the mechanics of the process. The goal is to obtain a clean infrared spectrum of the sample, free from interference.
Why Potassium Bromide?
Potassium bromide (KBr) is an alkali halide salt with two critical properties for this application. First, it is transparent to infrared radiation over a wide range (typically 4000 cm⁻¹ to 400 cm⁻¹), meaning it does not absorb IR light in the region of interest and won't interfere with the sample's spectrum.
Second, under high pressure, KBr crystals exhibit plastic deformation. This allows the fine powder to fuse together into a solid, glass-like disc that holds the dispersed sample particles in a fixed matrix.
The Role of Sample Preparation
The quality of the final spectrum is entirely dependent on the quality of the pellet. The sample must be finely pulverized and thoroughly mixed with the KBr powder, typically at a ratio of about 1:100 (sample to KBr).
This fine grinding is crucial to reduce light scattering. Large sample particles can scatter the infrared beam, causing a distorted, sloping baseline in the resulting spectrum and making interpretation difficult. The ideal is a homogenous mixture with particles smaller than the wavelength of the IR light being used.
The Function of Pressure and Vacuum
Once the sample-KBr mixture is placed in a die, it is pressed in a hydraulic press at a load of several tons. This immense pressure causes the KBr to cold-flow and encapsulate the sample particles, forming the transparent disc.
Most pellet presses operate under vacuum. This step is critical for removing trapped air and, more importantly, atmospheric moisture. Water (H₂O) has very strong IR absorption bands that can easily obscure important spectral features of the sample. An inadequate vacuum results in cloudy, brittle pellets that scatter light and show significant water contamination.
Understanding the Trade-offs and Common Pitfalls
While powerful, the KBr pellet method requires careful technique to avoid generating poor or misleading data. Understanding the potential issues is key to mastering the method.
Moisture Contamination
This is the most common problem. KBr is hygroscopic, meaning it readily absorbs moisture from the air. The KBr powder must be oven-dried (e.g., at 110 °C for 2-3 hours) and stored in a desiccator. All preparation steps should be performed as quickly as possible to minimize exposure to humid air.
Inconsistent Particle Size
If the sample is not ground finely enough, or if it re-agglomerates, the resulting spectrum will suffer from light scattering. This appears as a rising baseline toward higher wavenumbers, which can obscure weak absorption bands.
Pellet Imperfections
Cloudy or opaque pellets are a clear sign of problems. This is often caused by insufficient pressure, trapped moisture, or KBr powder that was not ground finely enough. Cracked or easily broken pellets indicate an inadequate vacuum was pulled during pressing.
Chemical and Physical Changes
The high pressure used to form the pellet can sometimes induce a polymorphic transformation in crystalline samples, changing their crystal structure and thus their IR spectrum. Additionally, excessive heat during the drying of KBr can cause it to oxidize to potassium bromate (KBrO₃), which can interfere with the analysis.
Making the Right Choice for Your Analysis
Properly executed, the KBr pellet technique provides high-quality spectral data for a vast range of solid samples. Your specific goal will determine which procedural details to prioritize.
- If your primary focus is qualitative identification: Your main goal is a clean, artifact-free spectrum. Prioritize using bone-dry KBr and ensuring the pellet is perfectly clear to get an unambiguous spectral fingerprint of your material.
- If your primary focus is quantitative analysis: Consistency is everything. Use a precise sample-to-KBr ratio for every pellet and apply the exact same pressure for the same duration to ensure pellet thickness and sample concentration are reproducible.
- If your sample is sensitive to pressure or moisture: The KBr pellet method may not be suitable. Consider alternative IR sampling techniques like Attenuated Total Reflectance (ATR) or preparing a Nujol mull.
Ultimately, the KBr pellet is a powerful method for turning an opaque solid into a measurable sample for infrared analysis, provided the practitioner remains vigilant against contamination and physical imperfections.
Summary Table:
| Key Aspect | Description |
|---|---|
| Primary Use | Sample preparation for Fourier Transform Infrared (FTIR) Spectroscopy of solid materials. |
| Core Principle | Disperses a solid sample within an IR-transparent potassium bromide (KBr) matrix. |
| Key Requirement | High-pressure pressing, often under vacuum, to form a clear, measurable disc. |
| Common Challenge | Moisture contamination, which can obscure the sample's IR spectrum. |
| Ideal For | Qualitative identification and quantitative analysis of a wide range of solids. |
Achieve precise and reliable FTIR results with expert sample preparation. The KBr pellet method is fundamental for accurate material analysis. KINTEK specializes in high-quality lab equipment and consumables, including reliable pellet presses and pure KBr powder, to support your laboratory's spectroscopic needs. Contact our experts today to discuss the right tools for your application and ensure your analyses are free from contamination and artifacts.
Get in touch via our Contact Form to enhance your lab's capabilities.
Visual Guide
Related Products
- Laboratory Hydraulic Press Split Electric Lab Pellet Press
- kbr pellet press 2t
- Automatic Laboratory Hydraulic Pellet Press Machine for Lab Use
- Laboratory Manual Hydraulic Pellet Press for Lab Use
- Laboratory Hydraulic Press Lab Pellet Press for Button Battery
People Also Ask
- What machine is used to make pellets? The Complete Guide to Pellet Mills & Production Systems
- What function does a laboratory hydraulic press perform in the preparation of solid-state battery electrolyte layers?
- What role does a laboratory hydraulic press play during cold-pressing? Optimize Your Vacuum Hot-Pressing Results
- What role does a uniaxial hydraulic press play in the fabrication of NaSICON ceramic cylinders? Pre-forming Excellence
- Why is a precision laboratory hydraulic press required for manufacturing molybdenum target green bodies?
- What happens if hydraulic pressure is too high? Prevent Catastrophic System Failure and Downtime
- Is there a future for hydraulics? Evolving Beyond Oil and Wires for Smart, High-Power Applications
- How can I make my hydraulic system more efficient? Slash Energy Costs and Reduce Heat Generation



















