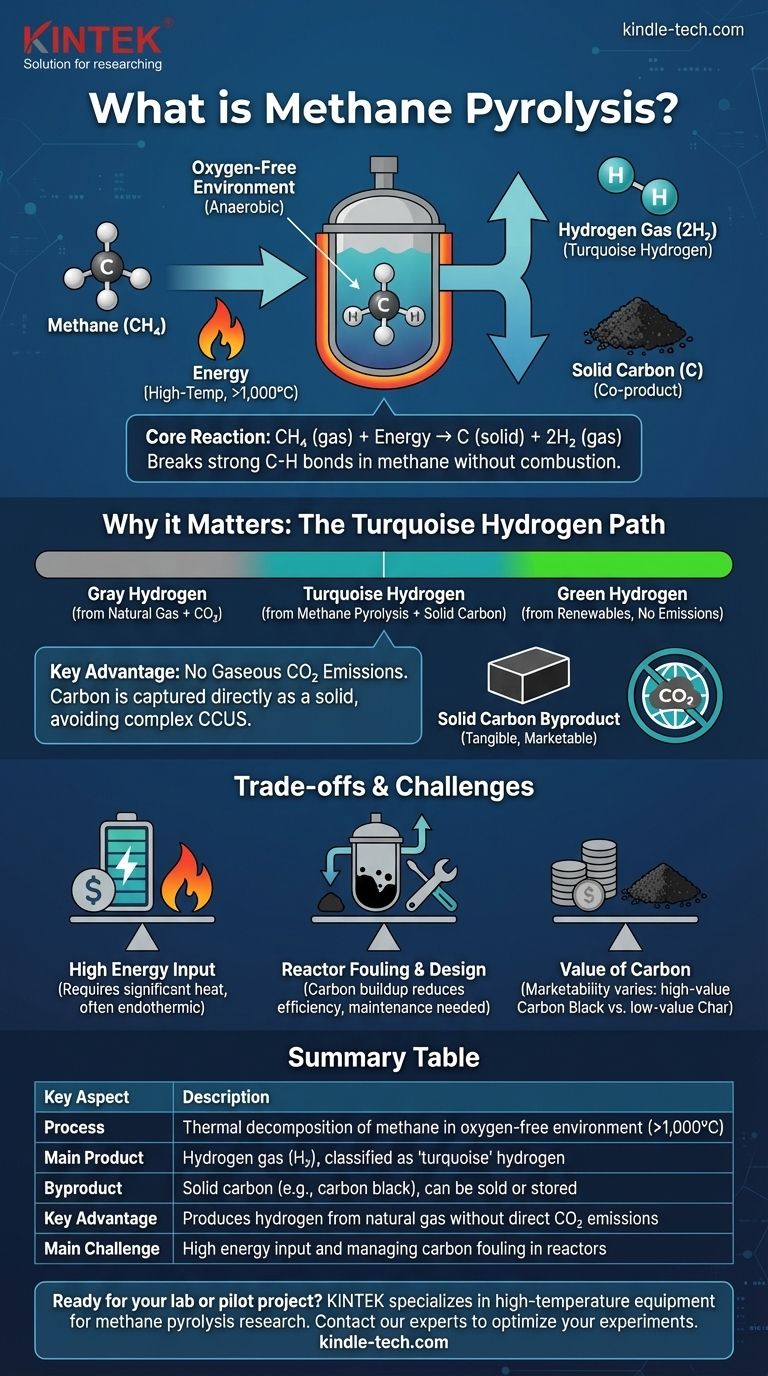
In the simplest terms, methane pyrolysis is a chemical process that breaks down methane (CH₄) into its fundamental components: hydrogen gas (H₂) and solid carbon (C). It achieves this by heating methane to very high temperatures in an oxygen-free environment. This absence of oxygen is critical, as it prevents the methane from burning and instead forces it to decompose.
Methane pyrolysis is not just a chemical reaction; it is a strategy for producing valuable hydrogen from natural gas without releasing carbon dioxide into the atmosphere. The carbon is captured in a solid, manageable form, fundamentally changing the emissions profile of hydrogen production from fossil fuels.

The Core Chemical Reaction
The elegance of methane pyrolysis lies in its directness. The process, also known as methane cracking or decomposition, relies on thermal energy to break one of the strongest single bonds in organic chemistry.
The Fundamental Equation
The reaction is governed by a simple, clean equation: CH₄ (gas) + Energy → C (solid) + 2H₂ (gas). One molecule of methane yields one atom of solid carbon and two molecules of hydrogen gas.
The Role of High Temperature
Breaking the stable carbon-hydrogen bonds in methane requires a significant energy input, making the process endothermic. This is typically achieved by heating the methane to temperatures above 1,000°C (1,832°F), though catalysts can sometimes lower this requirement.
The Absence of Oxygen
The entire process must occur in an anaerobic (oxygen-free) environment. If oxygen were present, the methane would combust, producing carbon dioxide (CO₂) and water (H₂O) instead of the desired hydrogen and solid carbon.
Why Pyrolysis Matters for Hydrogen Production
Methane pyrolysis is gaining significant attention as a potential bridge technology in the energy transition. It offers a unique value proposition compared to other established methods of hydrogen production.
A "Turquoise" Hydrogen Pathway
Hydrogen is often color-coded by its production method. Gray hydrogen is made from natural gas via steam-methane reforming (SMR), a process that emits large amounts of CO₂. Green hydrogen is made via electrolysis using renewable electricity, with zero emissions.
Methane pyrolysis creates what is known as turquoise hydrogen. It uses a fossil fuel feedstock (methane) but does not produce gaseous carbon emissions, placing it between gray and green on the carbon-intensity spectrum.
The Key Advantage: No Gaseous Carbon Emissions
The defining benefit of pyrolysis is that the carbon is captured directly as a solid. This avoids the need for complex and expensive Carbon Capture, Utilization, and Storage (CCUS) systems, which are required to convert gray hydrogen into lower-emission "blue" hydrogen.
The Solid Carbon Co-Product
Unlike in other processes where CO₂ is a waste product to be managed, the solid carbon from pyrolysis is a tangible co-product. Its form, purity, and market value are critical to the overall economics of the process.
Understanding the Trade-offs and Challenges
While promising, methane pyrolysis is not a silver bullet. Its viability depends on solving significant technical and economic challenges.
High Energy Input
As an endothermic process, pyrolysis demands a large, continuous supply of high-temperature heat. The source of this energy is a critical factor. If the heat is generated by burning more natural gas, the overall carbon footprint of the process increases, diminishing its "low-carbon" credentials. Using renewable electricity for plasma or resistive heating is a solution, but it adds cost.
Reactor Design and Fouling
Managing the process at extreme temperatures is a major engineering challenge. A key issue is carbon fouling, where the solid carbon byproduct deposits on reactor surfaces, catalysts, and heat exchangers, reducing efficiency and requiring periodic shutdowns for cleaning.
The Value of the Carbon
The economic viability of turquoise hydrogen often hinges on the ability to sell the solid carbon co-product. The value of this carbon varies dramatically, from high-value carbon black used in tires and pigments to low-value carbon char or soot that may even have a disposal cost. Producing a consistent, high-purity carbon product is a primary technical goal.
Making the Right Choice for Your Goal
Your assessment of methane pyrolysis technology must be aligned with your specific strategic objectives.
- If your primary focus is decarbonizing existing natural gas infrastructure: Pyrolysis offers a compelling pathway to produce hydrogen without direct CO₂ emissions, potentially leveraging existing gas pipelines while avoiding the capital cost and geological risk of CCUS.
- If your primary focus is producing the absolute cleanest hydrogen: Green hydrogen, produced via electrolysis powered by dedicated renewable energy, remains the gold standard, as it eliminates the use of fossil fuels entirely.
- If your primary focus is on economic viability: A pyrolysis project's success is determined by three factors: access to cheap natural gas, a low-cost and low-carbon energy source for heat, and a reliable market for the high-value solid carbon produced.
Methane pyrolysis represents a powerful but complex tool for decarbonization, where managing the energy input and the carbon output is just as important as producing the hydrogen itself.
Summary Table:
| Key Aspect | Description |
|---|---|
| Process | Thermal decomposition of methane in an oxygen-free environment (>1,000°C). |
| Main Product | Hydrogen gas (H₂), classified as 'turquoise' hydrogen. |
| Byproduct | Solid carbon (e.g., carbon black), which can be sold or stored. |
| Key Advantage | Produces hydrogen from natural gas without direct CO₂ emissions. |
| Main Challenge | High energy input and managing carbon fouling in reactors. |
Ready to explore hydrogen production solutions for your lab or pilot project? KINTEK specializes in high-temperature laboratory equipment and consumables essential for researching and developing processes like methane pyrolysis. Whether you need reactors, heating elements, or analytical tools, our expertise can help you optimize your experiments and scale your technology. Contact our experts today to discuss how we can support your clean energy innovation.
Visual Guide
Related Products
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
People Also Ask
- What is atomic layer deposition of metals? Achieve Atomic-Scale Precision for Your Thin Films
- What is the function of a reaction vessel with controlled humidity in CVD? Master Silicone Nanofilament Growth
- What function does a horizontal tubular quartz reactor serve in a hot-wall CVD system? Core Performance & Role
- How thick is chemical vapor deposition? Achieve Precise Control from Nanometers to Micrometers
- What is the energy range of sputtering? From Threshold to Optimal Deposition
- On which factor properties of thin film varies? Master the Deposition Process for Optimal Performance
- What is the chemical Vapour deposition method for thin films? Build High-Purity, Conformal Coatings
- What are the techniques of vapor phase deposition? Choose Between PVD and CVD for Your Thin-Film Needs



















