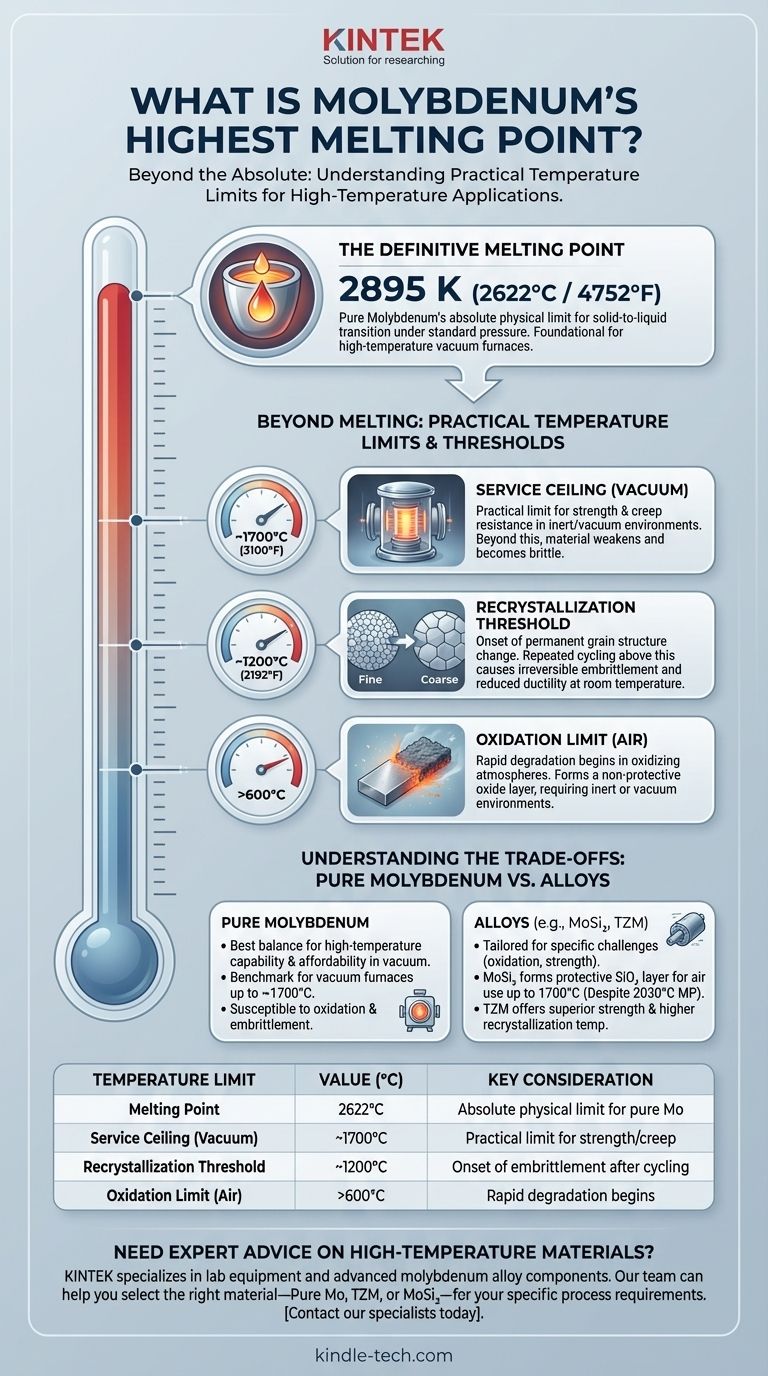
The definitive melting point of pure molybdenum is 2895 K, which translates to 2622°C or 4752°F. This exceptionally high value places it in the class of refractory metals, making it a foundational material for high-temperature industrial processes. However, this number only tells part of the story.
While molybdenum's high melting point is its defining characteristic, its practical usefulness in any given application is governed by lower temperature thresholds. Understanding the distinction between the melting point and the maximum service temperature is critical for engineering success.

The Core Property: Molybdenum's Melting Point
The Definitive Value
The accepted melting point for pure molybdenum is 2895 Kelvin (2622°C / 4752°F). This is the temperature at which the solid metal transitions into a liquid state under standard pressure.
Why This Matters for High-Temperature Applications
This high melting point is the primary reason molybdenum is used as a core structural material in applications where other metals would fail. This includes components like heating elements, crucibles, and structural supports inside high-temperature vacuum furnaces used for processes like smelting, brazing, and crystal growth.
Beyond Melting: Understanding Practical Temperature Limits
The melting point is a physical constant, but the maximum usable temperature is a practical limit that depends entirely on the operating environment and desired mechanical properties. Molybdenum can fail long before it melts.
The Recrystallization Threshold (~1200°C)
When molybdenum is heated above approximately 1200°C (2192°F), its internal grain structure changes. This process, called recrystallization, permanently reduces its ductility and makes the material significantly more brittle at room temperature. For applications requiring repeated heating and cooling, staying below this threshold is crucial to prevent mechanical failure.
The Service Ceiling in a Vacuum (~1700°C)
In an inert or vacuum environment, molybdenum can be used at temperatures up to 1700°C (3100°F). Beyond this point, it begins to lose its strength and becomes increasingly brittle, even if it is not exposed to oxygen. Creep resistance also diminishes, meaning it will slowly deform under its own weight.
The Oxidation Limit (Above ~600°C in Air)
Molybdenum's greatest weakness is its poor resistance to oxidation. In the presence of air, it will begin to rapidly oxidize at temperatures above 600°C. This forms a volatile oxide layer that does not protect the underlying metal, leading to rapid material loss. For this reason, pure molybdenum is almost exclusively used in vacuum or protective inert gas atmospheres.
Understanding the Trade-offs: Pure Molybdenum vs. Its Alloys
Alloying molybdenum doesn't necessarily increase its melting point, but it is often done to overcome its practical limitations, especially oxidation and high-temperature strength.
The Myth of "Higher is Always Better"
An excellent example is molybdenum disilicide (MoSi₂). This alloy has a melting point of only 2030°C, significantly lower than pure molybdenum. However, when heated in air, it forms a protective layer of silicon dioxide (SiO₂) that allows it to be used continuously at 1700°C for thousands of hours without degrading.
Enhancing Strength and Ductility
Alloys like TZM (titanium-zirconium-molybdenum) are designed to have superior strength and higher recrystallization temperatures than pure molybdenum. Adding elements like lanthanum or other rare earths can also improve ductility and creep resistance at extreme temperatures.
Cost vs. Performance
Pure molybdenum often provides the best balance of high-temperature capability and affordability for applications in a controlled vacuum environment. Specialized alloys offer superior performance for specific challenges, such as oxidation or mechanical stress, but typically at a higher cost.
Making the Right Choice for Your Application
Choosing the correct material requires looking beyond a single data point and considering the entire operational context.
- If your primary focus is maximum temperature resistance in a vacuum: Pure molybdenum is your benchmark, but be aware of mechanical degradation and embrittlement at service temperatures above 1700°C.
- If you are operating in an oxidizing (air) atmosphere: An alloy like molybdenum disilicide is far superior, despite its lower melting point, due to its self-healing protective oxide layer.
- If you need to maintain ductility after thermal cycling: You must operate below the recrystallization temperature of ~1200°C to avoid irreversible embrittlement.
- If you require maximum high-temperature strength and creep resistance: Consider a specialized alloy like TZM, which is engineered for better mechanical stability at elevated temperatures.
Ultimately, selecting the right molybdenum material depends less on its absolute melting point and more on the specific operating environment and mechanical demands of your process.
Summary Table:
| Temperature Limit | Value (°C) | Key Consideration |
|---|---|---|
| Melting Point | 2622°C | Absolute physical limit for pure Mo |
| Service Ceiling (Vacuum) | ~1700°C | Practical limit for strength/creep |
| Recrystallization Threshold | ~1200°C | Onset of embrittlement after cycling |
| Oxidation Limit (Air) | >600°C | Rapid degradation begins |
Need expert advice on high-temperature materials for your lab or industrial furnace?
At KINTEK, we specialize in lab equipment and consumables, including molybdenum and advanced alloy components for demanding thermal applications. Our team can help you select the right material—whether pure molybdenum, TZM alloy, or MoSi₂—based on your specific temperature, atmosphere, and mechanical requirements.
Contact our specialists today to optimize your high-temperature process performance and durability.
Visual Guide
Related Products
- Molybdenum Vacuum Heat Treat Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering Brazing Furnace
- Laboratory High Pressure Vacuum Tube Furnace
People Also Ask
- What are the most commonly used metals in a vacuum furnace's hot zone? Discover the Key to High-Purity Processing
- What are the safety precautions in a heat treatment process? A Guide to Engineering, Administrative, and PPE Controls
- How can the temperature rise of a furnace be reduced if it is too high? Fix Airflow Issues for Safe & Efficient Heating
- Is heat Cannot travel in a vacuum True or false? Discover How Heat Crosses the Void of Space
- Why is high-temperature vacuum heat treatment critical for Cr-Ni steel? Optimize Strength & Surface Integrity



















