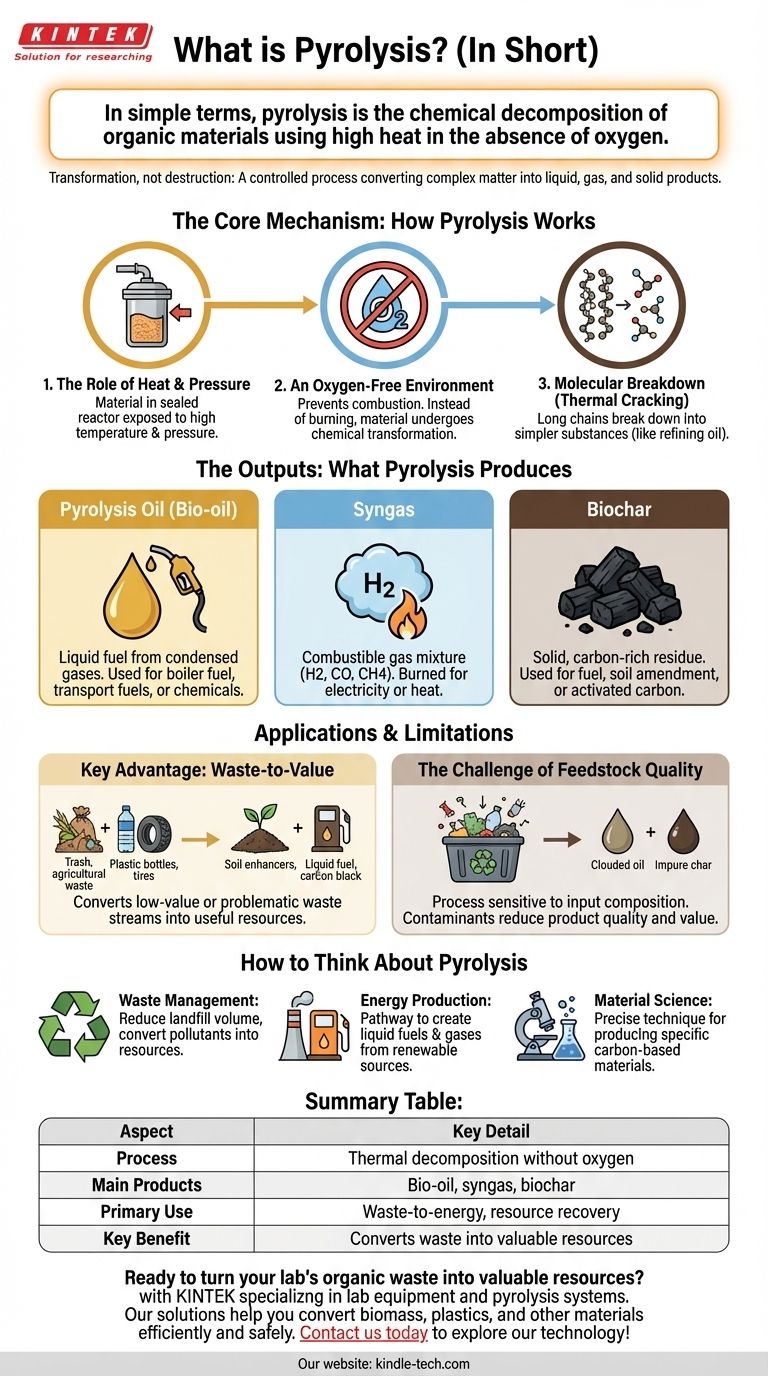
In simple terms, pyrolysis is the chemical decomposition of organic materials using high heat in the absence of oxygen. Rather than burning the material, which requires oxygen, pyrolysis uses heat to break down large, complex molecules into smaller, simpler, and often more valuable substances.
The core function of pyrolysis is transformation, not destruction. It's a controlled process that uses heat to convert complex organic matter, like waste plastic or biomass, into a mix of liquid fuel (bio-oil), combustible gas (syngas), and solid char (biochar).

The Core Mechanism: How Pyrolysis Works
Pyrolysis is fundamentally different from incineration or combustion because it carefully controls the chemical environment to achieve a specific outcome.
The Role of Heat and Pressure
The process begins by feeding material into a sealed reactor. Inside, it is exposed to controlled, high temperatures. This intense heat causes pressure to build and provides the energy needed for chemical bonds to break apart.
An Oxygen-Free Environment
This is the most critical element of pyrolysis. By starving the reactor of oxygen, combustion is prevented. Instead of burning and releasing its energy as heat and light, the material undergoes a chemical transformation.
Molecular Breakdown (Thermal Cracking)
As the material heats up, the long, complex molecular chains within it become unstable and break down into smaller, lighter molecules. This process is often compared to the thermal cracking used in petroleum refining to produce gasoline from crude oil.
The Outputs: What Pyrolysis Produces
The specific output depends heavily on the input material and process conditions, but it generally yields three primary products.
Pyrolysis Oil (Bio-oil)
This is a liquid fuel that results from the condensation of pyrolytic gases. Depending on its purity, this bio-oil can be used as a boiler fuel, upgraded into transportation fuels, or used as a source of industrial chemicals.
Syngas
This is a mixture of combustible gases, primarily hydrogen, carbon monoxide, and methane. Syngas can be burned to generate electricity or heat for the pyrolysis process itself, making it partially self-sustaining.
Biochar
Biochar is the solid, stable, carbon-rich residue left behind. A familiar example of this is charcoal produced from wood. It can be burned as a fuel, used as a soil amendment to improve fertility, or processed into high-value activated carbon.
Understanding the Applications and Limitations
While powerful, pyrolysis is not a universal solution. Its effectiveness is tied directly to its application and the quality of the feedstock.
Key Advantage: Waste-to-Value
The primary driver for pyrolysis is its ability to convert low-value or problematic waste streams into valuable products. This includes turning agricultural waste into soil enhancers, non-recyclable plastics into liquid fuel, and tires into carbon black and oil.
The Challenge of Feedstock Quality
The process is highly sensitive to the composition of the input material. Contaminants in waste streams can end up in the final products, reducing their quality and value or requiring expensive post-processing steps to clean them up.
How to Think About Pyrolysis
Your perspective on pyrolysis will depend entirely on your goal.
- If your primary focus is waste management: See pyrolysis as a method to dramatically reduce landfill volume and convert potential pollutants into useful resources.
- If your primary focus is energy production: View pyrolysis as a pathway to create liquid fuels and combustible gases from renewable biomass or plastic waste.
- If your primary focus is material science: Understand pyrolysis as a precise technique for producing specific carbon-based materials, like biochar and activated carbon.
Ultimately, pyrolysis is a powerful chemical tool that reshapes our concept of waste by transforming it into a valuable starting material.
Summary Table:
| Aspect | Key Detail |
|---|---|
| Process | Thermal decomposition without oxygen |
| Main Products | Bio-oil, syngas, biochar |
| Primary Use | Waste-to-energy, resource recovery |
| Key Benefit | Converts waste into valuable resources |
Ready to turn your lab's organic waste into valuable resources? KINTEK specializes in lab equipment and consumables, including pyrolysis systems tailored for laboratory-scale waste transformation. Our solutions help you convert biomass, plastics, and other materials into bio-oil, syngas, and biochar efficiently and safely. Contact us today to explore how our pyrolysis technology can enhance your research and sustainability goals!
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Calciner Small Rotary Kiln Rotating Furnace
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Electric Rotary Kiln Small Rotary Furnace for Activated Carbon Regeneration
People Also Ask
- What are the negative effects of plastic pyrolysis? The Hidden Environmental and Health Risks
- What is the difference between refining and calcination? A Guide to Material Processing Stages
- What are the three stages of pyrolysis? Turn Waste into Biochar, Bio-Oil & Syngas
- Which is better pyrolysis or gasification? Choose the Right Process for Your Energy Goals
- What are the requirements for pyrolysis? Control Temperature and Atmosphere for Your Desired Product
- What are the types of pyrolysis reactors used in industry? Choose the Right Technology for Your Product
- What is a small pilot scale pyrolysis reactor? Your Guide to Choosing the Right Reactor Design
- What are the benefits of a plastic pyrolysis plant? Turn Waste Plastic into Valuable Resources



















