In short, electron beam (e-beam) sterilization offers a uniquely powerful combination of speed, material compatibility, and logistical simplicity. It is an FDA-approved method that uses electricity to generate high-energy electrons, providing rapid, on-demand sterilization without the significant material degradation or radioactive sources associated with older methods.
E-beam's primary advantage is not just one feature, but its unique balance of benefits. It delivers the speed and efficiency needed for modern supply chains while simultaneously protecting the integrity of sensitive materials, a combination that traditional methods often cannot match.

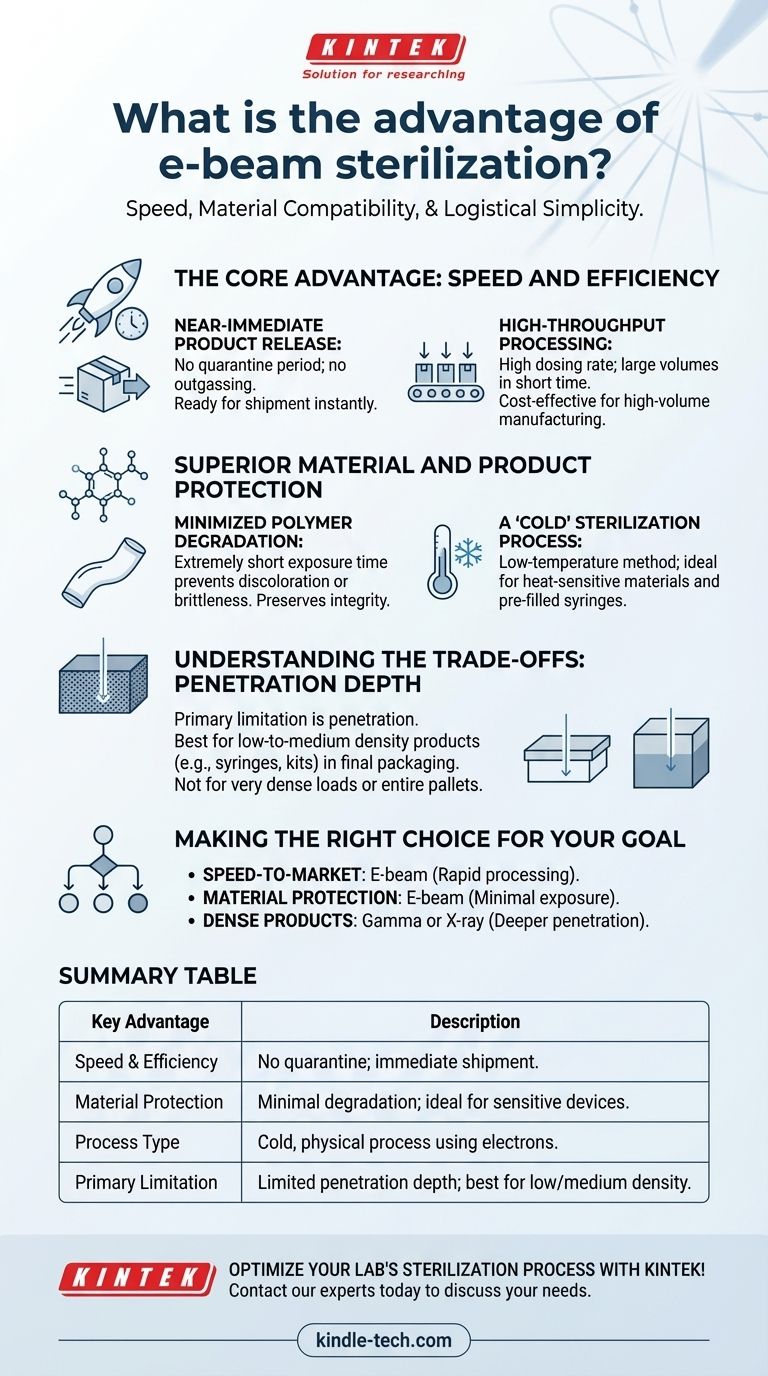
The Core Advantage: Speed and Efficiency
One of the most compelling reasons to choose e-beam is its incredible processing speed. This directly translates to significant improvements in supply chain agility and cost-effectiveness.
Near-Immediate Product Release
Unlike gas-based methods like Ethylene Oxide (EtO), e-beam sterilization requires no quarantine period for outgassing. Products are fully sterilized and ready for immediate shipment once the process is complete.
This is possible because e-beam is a physical process, not a chemical one. It uses electrons to break microbial DNA, achieving the required sterility assurance level (SAL) in a matter of seconds or minutes.
High-Throughput Processing
E-beam facilities operate with a very high dosing rate, meaning they can process a large volume of products in a short amount of time. This makes it an extremely cost-effective option for high-volume manufacturing.
The technology works like a light switch—it is on when needed and off when not, eliminating the standby time and continuous handling requirements of other methods.
Superior Material and Product Protection
For many modern products, especially in the medical device field, preserving the physical properties of the materials is just as important as achieving sterility.
Minimized Polymer Degradation
Many plastics and polymers can be damaged by other forms of irradiation, like gamma rays. This can result in discoloration, brittleness, or changes in physical properties.
E-beam's extremely short exposure time (seconds vs. hours for gamma) significantly reduces these effects. This preserves the integrity, function, and aesthetic quality of the final product, which is critical for complex medical devices.
A "Cold" Sterilization Process
The e-beam process imparts very little thermal energy to the product. While the core temperature may rise slightly, it remains a fundamentally low-temperature method.
This makes it ideal for sterilizing heat-sensitive materials, pre-filled syringes, or combination products that would be damaged by heat-based sterilization.
Understanding the Trade-offs
No technology is a universal solution. While e-beam's advantages are significant, its primary limitation is penetration depth.
The Impact of Product Density
Electron beams have less penetrating power than gamma or X-rays. This makes e-beam exceptionally well-suited for low-to-medium density products in their final packaging, such as syringes, surgical kits, and labware.
However, it is generally not suitable for very dense products or for sterilizing entire pallets of product at once. The configuration and density of the load must be carefully validated to ensure the electrons can penetrate fully and deliver the required dose to all parts of the product.
The Role of Competing Technologies
For extremely dense or complex product loads, X-ray sterilization offers similar benefits to e-beam (fast processing, no radioactive source) but with much deeper penetration, comparable to gamma.
Gamma irradiation, which uses a physical Cobalt-60 source, remains the choice for the most dense and challenging product loads due to its superior penetration, despite its slower processing time and the logistical complexities of handling radioactive material.
Making the Right Choice for Your Goal
Selecting the right sterilization method requires aligning the technology's strengths with your primary goal.
- If your primary focus is speed-to-market and supply chain agility: E-beam is the superior choice due to its rapid processing and immediate product release.
- If your primary focus is protecting sensitive polymers and materials: E-beam's minimal exposure time offers the best protection against material degradation.
- If your primary focus is sterilizing very dense or irregularly shaped products: Gamma or X-ray irradiation are likely better options due to their deeper penetration capabilities.
By understanding these core capabilities, you can confidently determine if e-beam's unique combination of speed and material protection aligns with your product's specific sterilization needs.
Summary Table:
| Key Advantage | Description |
|---|---|
| Speed & Efficiency | No quarantine period; products are ready for immediate shipment. |
| Material Protection | Minimal polymer degradation; ideal for sensitive medical devices. |
| Process Type | Cold, physical process using high-energy electrons. |
| Primary Limitation | Limited penetration depth, best for low-to-medium density products. |
Optimize your lab's sterilization process with KINTEK!
E-beam technology offers a powerful solution for labs needing fast, reliable, and material-friendly sterilization. If you work with sensitive medical devices, pharmaceuticals, or lab consumables, e-beam can enhance your supply chain agility and protect your product integrity.
As a specialist in lab equipment and consumables, KINTEK can help you determine if e-beam is the right choice for your specific needs. Contact our experts today to discuss how we can support your laboratory's sterilization and efficiency goals.
Visual Guide
Related Products
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
- Laboratory Sterilizer Lab Autoclave Vertical Pressure Steam Sterilizer for Liquid Crystal Display Automatic Type
- Laboratory Test Sieves and Sieving Machines
- Three-dimensional electromagnetic sieving instrument
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
People Also Ask
- What is a lab autoclave? Your Guide to Sterilization with Pressurized Steam
- Do you need to autoclave glassware? A Guide to Sterilization vs. Cleaning
- Why is it important to autoclave the prepared reagents before using? Ensure Sterility and Reliable Results
- What is the use of autoclave in medical? The Critical Role of Sterilization in Patient Safety
- Can autoclave sterilize liquid? Master Safe and Effective Liquid Sterilization



















