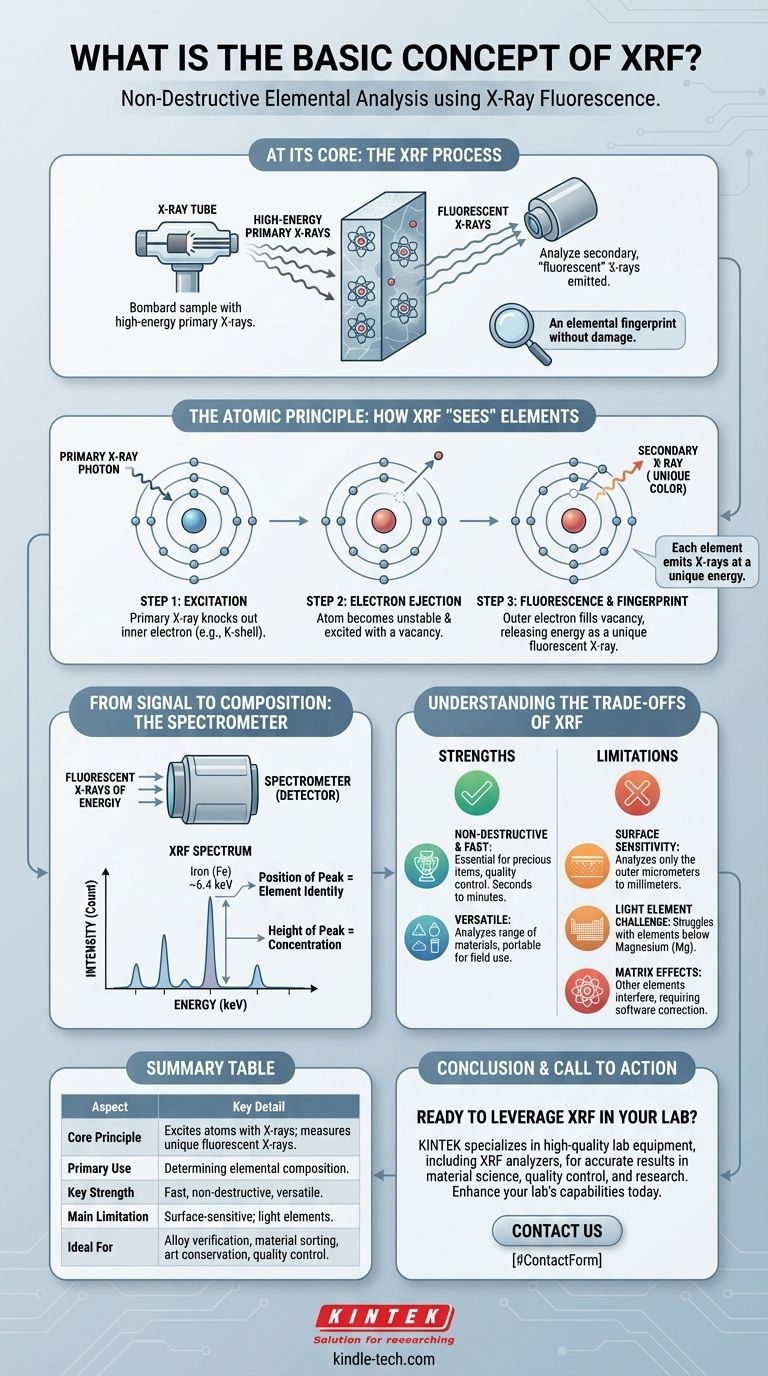
At its core, X-Ray Fluorescence (XRF) is a powerful and non-destructive analytical technique used to determine the elemental composition of a material. It works by bombarding a sample with high-energy X-rays and then analyzing the secondary, "fluorescent" X-rays that the sample emits in response. Because each chemical element emits fluorescent X-rays at a unique energy, this process acts as an elemental fingerprint, revealing exactly what the material is made of without damaging it.
XRF provides a fast, non-destructive way to "see" the elemental makeup of a material. It uses an external X-ray source to make a sample's atoms momentarily unstable, and it identifies the elements by measuring the unique energy signatures they release as they return to a stable state.

The Atomic Principle: How XRF "Sees" Elements
To understand XRF, you must visualize what happens at the atomic level. The process is a rapid, three-step chain reaction within the atoms of your sample.
Step 1: Excitation
An XRF instrument begins by directing a beam of primary X-rays, generated by an X-ray tube, onto the surface of the sample. This initial beam is powerful enough to penetrate the electron clouds of the atoms within the material.
Step 2: Electron Ejection
When a primary X-ray photon strikes an atom with sufficient energy, it can knock an electron out of one of its inner orbital shells (most commonly the innermost 'K' shell). This creates a vacancy, leaving the atom in an unstable, high-energy "excited" state.
Step 3: Fluorescence and the Elemental Fingerprint
This unstable state is corrected almost instantaneously. An electron from a higher-energy outer shell (such as the 'L' or 'M' shell) immediately drops down to fill the vacancy in the inner shell.
As the electron drops to a lower energy level, the excess energy is released in the form of a secondary X-ray. This emitted X-ray is called a fluorescent X-ray, and its energy is equal to the difference between the two orbital shells.
This energy difference is the crucial part. It is characteristic and unique for every single element. An iron atom will always release a fluorescent X-ray at a different, predictable energy than a nickel atom or a gold atom.
From Signal to Composition: The Role of the Spectrometer
Identifying these characteristic X-rays is the job of the spectrometer, which captures the fluorescent signals and translates them into compositional data.
Capturing and Sorting the Signal
A detector within the instrument collects the fluorescent X-rays being emitted from the sample. The system then sorts these incoming X-rays based on their energy. This is most commonly done with an Energy Dispersive (EDXRF) spectrometer, which can process many different energies at once.
Reading the Spectrum
The result is a spectrum, which is a graph plotting X-ray intensity (the number of X-rays detected) against X-ray energy. This spectrum will show a series of peaks.
The position of each peak on the energy axis identifies which element is present. For example, a peak appearing at ~6.4 keV is the fingerprint for iron. The height or area of that peak is generally proportional to the concentration of that element in the sample.
Understanding the Trade-offs of XRF
While powerful, XRF is not a universal solution. Understanding its strengths and weaknesses is critical for interpreting results correctly.
Strength: Non-Destructive and Fast
The greatest advantage of XRF is that it does not damage or alter the sample. This is essential for analyzing precious items like jewelry or artifacts, or for quality control on finished products. Analyses are also very fast, often taking from seconds to a few minutes.
Strength: Versatility
XRF can be used on a wide range of materials, including solids, liquids, powders, and films. The availability of portable, handheld XRF analyzers allows for immediate analysis in the field, on a factory floor, or at a scrap yard.
Limitation: Surface Sensitivity
The primary X-rays only penetrate a finite depth into the sample—from a few micrometers to several millimeters, depending on the material's density. This means XRF is fundamentally a surface analysis technique. The results may not be representative of the bulk composition if the material is not homogeneous.
Limitation: Light Element Detection
Standard XRF analyzers struggle to detect very light elements (those with atomic numbers below magnesium, Mg). The characteristic X-rays from these elements have very low energy and are easily absorbed by the air or the instrument's detector window. Detecting them requires specialized equipment, often with a vacuum environment.
Pitfall: Matrix Effects
The presence of other elements in the sample (the "matrix") can interfere with the results. X-rays from one element can be absorbed or enhanced by another, skewing the perceived concentration. Accurate quantitative analysis requires sophisticated software corrections to compensate for these matrix effects.
Making the Right Choice for Your Goal
Ultimately, the suitability of XRF depends entirely on your analytical objective.
- If your primary focus is rapid material sorting and identification: XRF is an unmatched tool for alloy verification, scrap metal sorting, or screening products for restricted substances (RoHS compliance).
- If your primary focus is analyzing precious or unique items: The non-destructive nature of XRF makes it the default choice for geochemistry, archeometry, and art conservation.
- If your primary focus is high-accuracy bulk composition: XRF is excellent for homogeneous materials like cements or metals, but you must use proper sample preparation and calibration to correct for matrix effects.
- If your primary focus is detecting carbon, nitrogen, or oxygen: You will need to use a different analytical technique, such as combustion analysis or a specialized Wavelength Dispersive (WDXRF) system.
By understanding its core principles and limitations, you can effectively leverage XRF as a powerful tool for revealing the elemental world.
Summary Table:
| Aspect | Key Detail |
|---|---|
| Core Principle | Excites atoms with X-rays; measures unique fluorescent X-rays emitted. |
| Primary Use | Determining the elemental composition of a material. |
| Key Strength | Fast, non-destructive, and versatile for solids, liquids, and powders. |
| Main Limitation | Surface-sensitive; can struggle with very light elements (e.g., carbon). |
| Ideal For | Alloy verification, material sorting, art conservation, quality control. |
Ready to leverage the power of non-destructive elemental analysis in your lab?
KINTEK specializes in providing high-quality lab equipment, including XRF analyzers, to meet your specific analytical needs. Whether you are involved in material science, quality control, or research, our solutions are designed to deliver accurate and reliable results.
Contact us today using the form below to discuss how our expertise can enhance your laboratory's capabilities and efficiency. Let us help you find the perfect equipment to unlock the elemental secrets of your materials.
Visual Guide
Related Products
- Customizable XRD Sample Holders for Diverse Research Applications
- Laboratory Test Sieves and Vibratory Sieve Shaker Machine
- Metallographic Specimen Mounting Machine for Laboratory Materials and Analysis
- Three-dimensional electromagnetic sieving instrument
- Double Plate Heating Press Mold for Lab
People Also Ask
- Does higher heat capacity mean higher melting point? Unraveling the Critical Difference
- Why is an airtight sample holder with a beryllium window required for XRD of sulfide solid electrolytes?
- What affects melting point chemistry? A Guide to Molecular Forces and Lattice Energy
- What is the minimum sample required for XRD analysis? Optimize Your Material Analysis
- What are the factors that affect melting and boiling point? Unlock the Science of Phase Transitions















