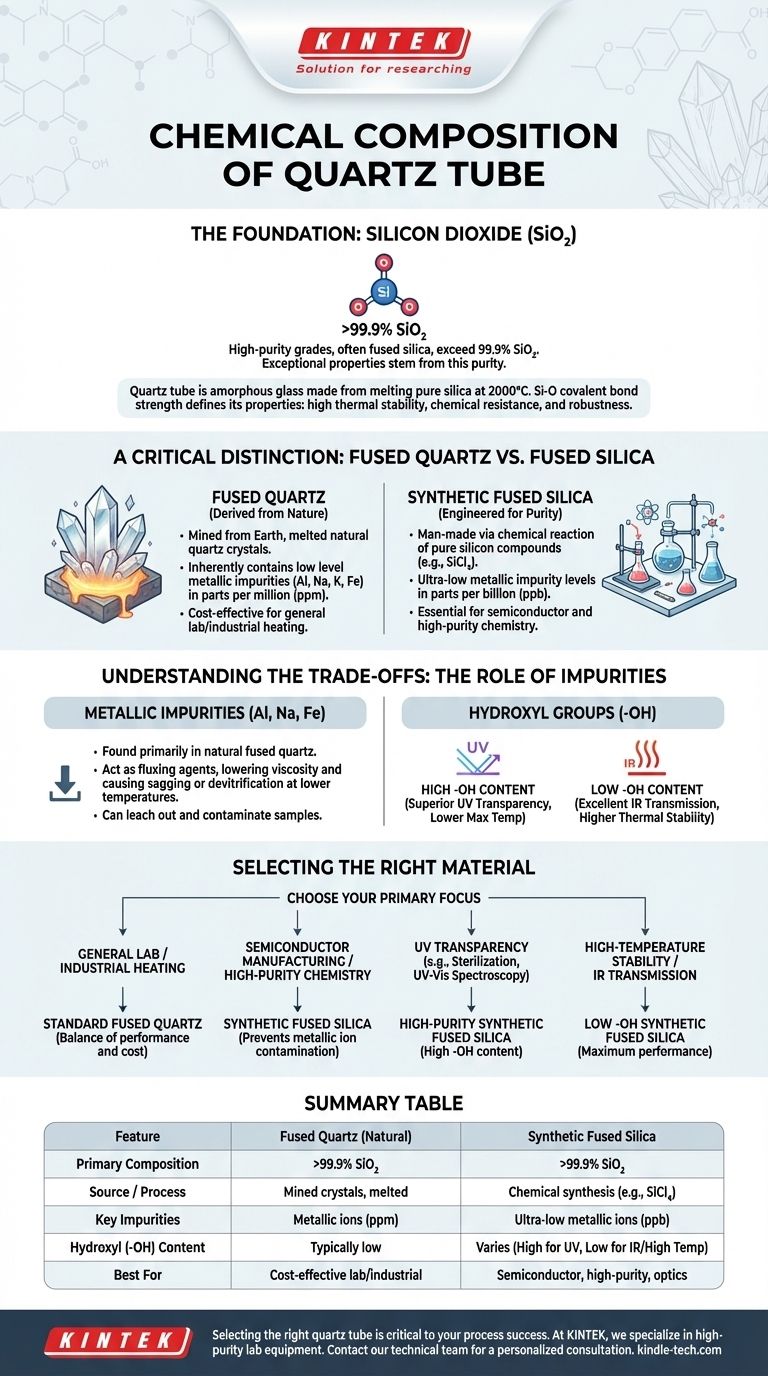
At its core, the chemical composition of a quartz tube is remarkably simple: it consists almost entirely of silicon dioxide (SiO₂). High-purity grades, often referred to as fused silica, exceed 99.9% SiO₂. This fundamental purity is the source of its exceptional properties, but the minute differences in trace elements and manufacturing methods are what truly define its performance in demanding applications.
The critical takeaway is not just that quartz tubes are made of silicon dioxide, but that the distinction between natural fused quartz and synthetic fused silica is paramount. The type and level of trace impurities—measured in parts per million or even billion—dictate the tube's thermal stability, optical transparency, and chemical purity, directly impacting its suitability for a specific technical role.

The Foundation: Silicon Dioxide (SiO₂)
Silicon dioxide is one of the most common chemical compounds on Earth, but creating a high-performance material from it requires immense energy and precision.
What is a Quartz Tube?
A "quartz tube" is technically a misnomer in most industrial contexts. The material is not crystalline quartz but rather an amorphous (non-crystalline) glass made from it.
This glassy state, known as fused quartz or fused silica, is produced by melting highly pure silica feedstock at temperatures around 2000°C and then cooling it into its final shape.
The Power of the Si-O Bond
The strength of the silicon-oxygen (Si-O) covalent bond is the origin of quartz's most valued characteristics.
This strong atomic structure is directly responsible for its high thermal stability, exceptional chemical resistance, and robust mechanical properties.
A Critical Distinction: Fused Quartz vs. Fused Silica
While often used interchangeably, these terms refer to two distinct grades of material with different manufacturing paths and purity levels. Understanding this difference is key to selecting the correct material.
Fused Quartz: Derived from Nature
Fused quartz is produced by melting naturally occurring, high-purity quartz crystals, typically mined from the earth.
Because its source is natural, fused quartz inherently contains a low level of metallic impurities. These often include aluminum (Al), sodium (Na), potassium (K), and iron (Fe), typically measured in parts per million (ppm).
Synthetic Fused Silica: Engineered for Purity
Synthetic fused silica is man-made through the chemical reaction of pure silicon compounds, such as silicon tetrachloride (SiCl₄). This process avoids the natural impurities found in mined crystals.
The result is a material with exceptionally low metallic impurity levels, often measured in parts per billion (ppb). This ultra-high purity is essential for applications like semiconductor manufacturing, where even trace metal contamination can ruin a process.
Understanding the Trade-offs: The Role of Impurities
The "impurities" in a quartz tube are not just unwanted contaminants; they are compositional variables that define the material's behavior.
Metallic Impurities (Al, Na, Fe)
These elements, primarily found in natural fused quartz, are the main limiting factor for high-temperature use. They act as fluxing agents, lowering the material's viscosity and causing it to sag or devitrify (recrystallize) at lower temperatures.
For high-purity chemical processes or semiconductor wafer processing, these metals can also leach out and contaminate the sample or environment.
Hydroxyl Groups (-OH)
Water, in the form of hydroxyl groups, is another critical "impurity." Its concentration depends on the manufacturing method.
Materials with high -OH content (often from flame hydrolysis synthesis) offer superior transparency in the deep ultraviolet (UV) spectrum. However, the -OH groups lower the material's maximum service temperature.
Conversely, materials with low -OH content (from plasma fusion) have excellent transmission in the infrared (IR) spectrum and higher thermal stability, making them ideal for fiber optics and high-temperature furnace tubes.
Selecting the Right Material for Your Application
Choosing the correct quartz tube is a matter of matching its specific composition to your primary technical goal.
- If your primary focus is general laboratory use or industrial heating: Standard fused quartz offers an excellent balance of performance and cost-effectiveness.
- If your primary focus is semiconductor manufacturing or high-purity chemistry: You must use synthetic fused silica to prevent metallic ion contamination.
- If your primary focus is UV transparency (e.g., sterilization lamps, UV-Vis spectroscopy): Select a high-purity synthetic fused silica with a high -OH content.
- If your primary focus is high-temperature stability or IR transmission: You need a low -OH synthetic fused silica for maximum performance.
Understanding these compositional nuances allows you to select not just a material, but the precise tool for your technical challenge.
Summary Table:
| Feature | Fused Quartz (Natural) | Synthetic Fused Silica |
|---|---|---|
| Primary Composition | >99.9% SiO₂ | >99.9% SiO₂ |
| Source / Process | Mined crystals, melted | Chemical synthesis (e.g., SiCl₄) |
| Key Impurities | Metallic ions (Al, Na, K, Fe) in ppm | Ultra-low metallic ions (ppb) |
| Hydroxyl (-OH) Content | Typically low | Varies (High for UV, Low for IR/High Temp) |
| Best For | Cost-effective lab/industrial heating | Semiconductor, high-purity chemistry, optics |
Selecting the right quartz tube is critical to your process success.
At KINTEK, we specialize in providing high-purity lab equipment and consumables. Our experts understand the nuanced differences between fused quartz and synthetic fused silica. We can help you select the perfect tube to ensure optimal performance for your specific application—whether it's semiconductor manufacturing requiring ultra-low metals, UV applications needing high-OH content, or high-temperature processes demanding low-OH stability.
Don't let material composition compromise your results. Contact our technical team today for a personalized consultation and get the precise quartz solution your laboratory needs.
Visual Guide
Related Products
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- Laboratory High Pressure Vacuum Tube Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
People Also Ask
- How should an oil-free diaphragm vacuum pump be maintained? A Proactive Guide to Maximize Pump Lifespan
- Why is a Back Pressure Regulator Necessary for High-Temp Systems? Ensure Liquid Stability & Prevent Boiling
- Why are cemented carbide jars and high-chromium alloy balls selected for CoCrCuFeNi HEA grinding?
- What are the functions of high-purity quartz balls and quartz sand for catalyst testing? Enhance Reactor Performance
- Why are PID temperature controllers and internal cooling systems essential for autohydrolysis? Precision & Quenching
- What is the purpose of installing a high-efficiency cold trap? Protect Sensors and Ensure Data Accuracy
- What role does an ultrasonic cleaner play in specimen pretreatment for supercritical water? Ensure Experimental Purity
- What is the function of a Cold Trap in a pervaporation-assisted membrane reactor process? Optimize Solvent Recovery



















