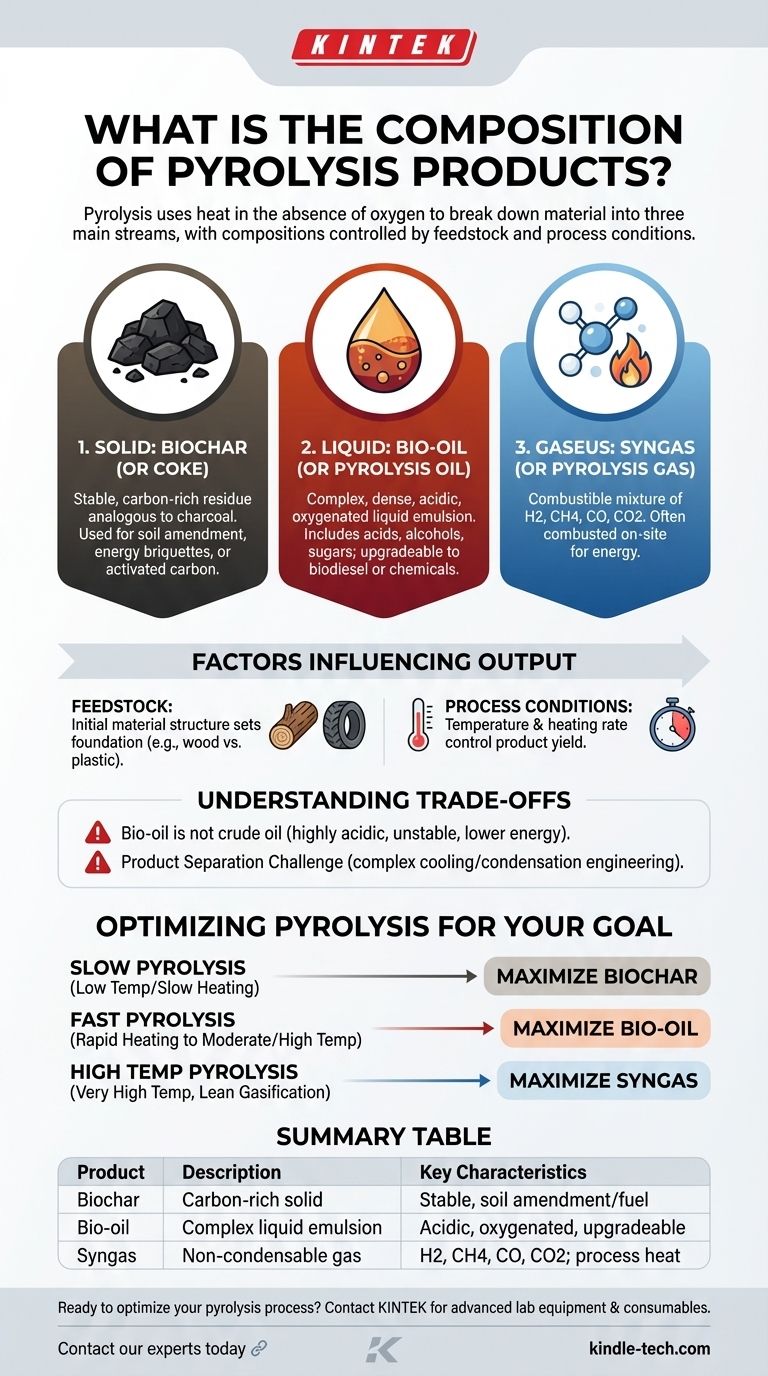
In short, pyrolysis breaks down a material using heat in the absence of oxygen to produce three distinct product streams: a solid, a liquid, and a gas. The solid is a carbon-rich substance called biochar or coke. The liquid is a complex mixture called bio-oil or pyrolysis oil. The gas is a combustible mixture of non-condensable gases often referred to as syngas.
The central takeaway is that pyrolysis does not produce a single, fixed output. The proportions and specific chemical makeup of the resulting char, oil, and gas are directly controlled by the initial feedstock and the specific process conditions, particularly temperature and heating rate.

The Three Core Products of Pyrolysis
Pyrolysis is a thermochemical decomposition process that results in the irreversible chemical and physical transformation of the initial material. This process yields three primary products in varying ratios.
The Solid Fraction: Biochar (or Coke)
Biochar is the stable, carbon-rich solid that remains after the volatile components of the feedstock have been driven off. It is analogous to charcoal.
This solid product is valued for its use in agriculture as a soil amendment, as a material for creating energy briquettes, or as an activated carbon for filtration and sorption.
The Liquid Fraction: Bio-oil (or Pyrolysis Oil)
As the process gas cools, a complex liquid known as bio-oil condenses out. This is not a simple oil but a dense, acidic, and oxygenated liquid emulsion.
Its composition includes hundreds of organic compounds, such as acids, alcohols, phenols, and sugars, along with a significant amount of water. This liquid can be upgraded into transportation fuels like biodiesel or serve as a source for specialty chemicals.
The Gaseous Fraction: Syngas (or Pyrolysis Gas)
The components that do not condense into a liquid form the gaseous product, often called syngas or pyrolysis gas.
This is a mixture of combustible and non-combustible gases, including hydrogen (H2), methane (CH4), carbon monoxide (CO), and carbon dioxide (CO2). In most industrial applications, this gas is immediately combusted on-site to provide the heat required to sustain the pyrolysis reaction, making the process more energy-efficient.
Why Product Composition Varies
You can't expect the same output from pyrolyzing wood as you would from pyrolyzing plastic. The final product mix is a direct function of both the starting material and how you run the process.
The Role of Feedstock
The chemical structure of the initial material—known as the feedstock—sets the foundation for the final products.
A biomass feedstock like wood or straw, which is high in cellulose and lignin, will produce a different balance of bio-oil and char than a feedstock like waste tires, which is based on hydrocarbon polymers.
The Impact of Process Conditions
The most critical factor in determining the product yield is the set of process conditions, primarily temperature and heating rate.
By controlling these variables, you can steer the process to favor one type of product over another. This is the key to tuning the output for a specific goal.
Understanding the Trade-offs
While pyrolysis is a powerful conversion technology, it's essential to understand its practical limitations and complexities.
Bio-oil is Not Crude Oil
A common misconception is that bio-oil is a direct replacement for crude oil. It is not.
Bio-oil is highly acidic, corrosive, and chemically unstable. It contains significant amounts of water and oxygen, giving it a lower energy density. It requires substantial and costly upgrading before it can be used in conventional engines or refineries.
The Challenge of Product Separation
The output of a pyrolysis reactor is a hot mixture of gases and vapors. This stream must be carefully managed through cooling and condensation stages to effectively separate the liquid bio-oil from the non-condensable syngas.
This separation and collection system adds significant engineering complexity and cost to a pyrolysis plant.
Optimizing Pyrolysis for Your Goal
By manipulating the process conditions, you can tailor the output to meet a specific objective. This transforms pyrolysis from a simple decomposition process into a versatile production platform.
- If your primary focus is producing a soil amendment or solid fuel: Use slow pyrolysis, which involves lower temperatures and slower heating rates, to maximize the yield of biochar.
- If your primary focus is creating a liquid biofuel or chemical feedstock: Use fast pyrolysis, with very rapid heating to moderate-to-high temperatures, to maximize the yield of bio-oil.
- If your primary focus is generating combustible gas for energy: Use very high temperatures in a process that leans toward gasification to maximize the yield of syngas.
By understanding these principles, you can view pyrolysis not as a single process, but as a flexible tool for converting diverse feedstocks into valuable resources.
Summary Table:
| Product | Description | Key Characteristics |
|---|---|---|
| Biochar (Solid) | Carbon-rich solid residue | Stable, used for soil amendment, filtration, or fuel |
| Bio-oil (Liquid) | Complex liquid emulsion from condensed vapors | Acidic, oxygenated, can be upgraded to biofuels or chemicals |
| Syngas (Gas) | Non-condensable gas mixture | Contains H2, CH4, CO, CO2; often used for process heat |
Ready to optimize your pyrolysis process for maximum yield and efficiency? KINTEK specializes in advanced lab equipment and consumables for pyrolysis research and development. Whether you're focused on biochar production, bio-oil upgrading, or syngas utilization, our tailored solutions help you achieve precise control over process conditions and product outcomes. Contact our experts today to discuss how we can support your specific laboratory needs and accelerate your pyrolysis projects!
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
People Also Ask
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas
- What are the reactions involved in pyrolysis of biomass? Unlock the Chemistry for Tailored Bio-Products
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs
- Is pyrolysis viable? A Guide to Economic, Technological, and Environmental Success



















