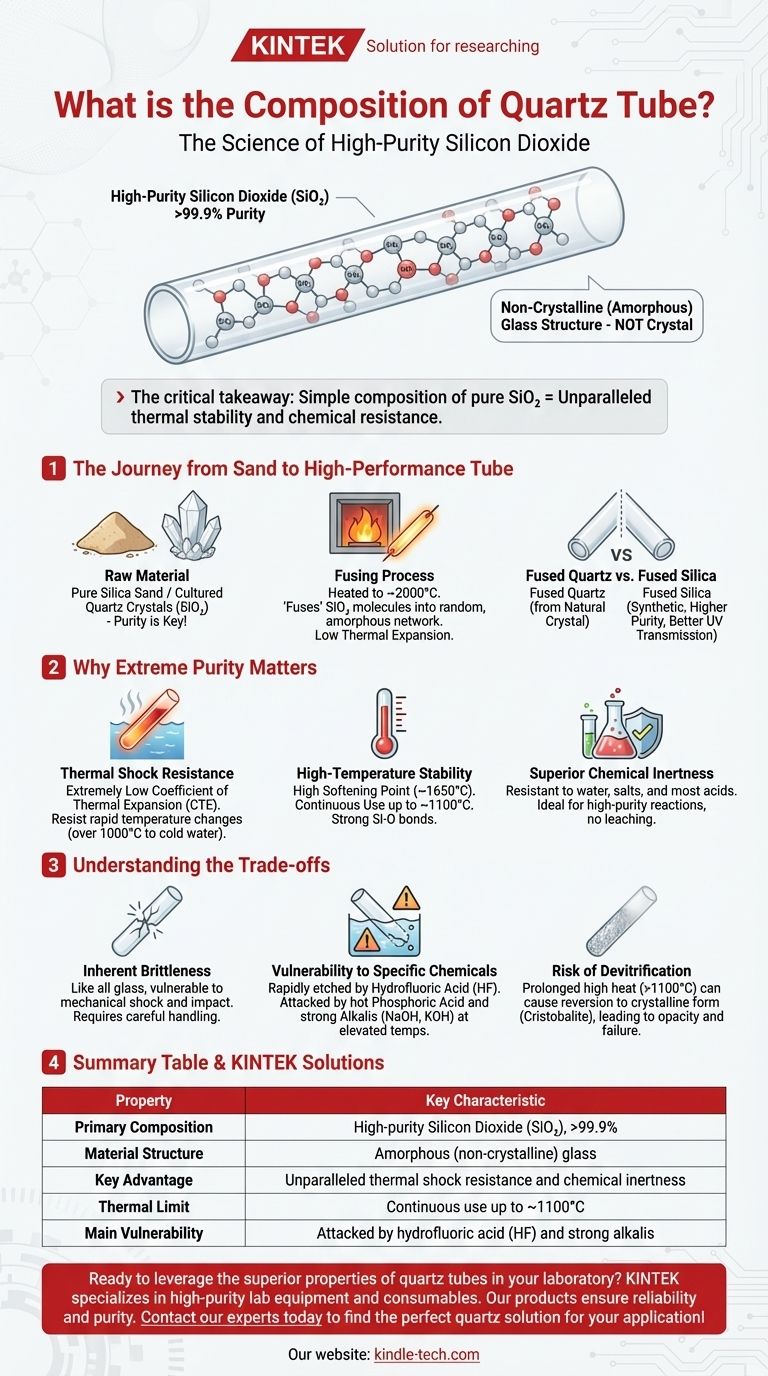
At its core, a quartz tube is composed of high-purity silicon dioxide (SiO₂), often exceeding 99.9% purity. This simple, non-crystalline (amorphous) glass structure is fundamentally different from natural quartz crystal. It is this extreme purity and unique atomic arrangement that gives the material its exceptional performance characteristics for industrial and scientific applications.
The critical takeaway is not just what a quartz tube is made of, but why its simple composition of pure silicon dioxide (SiO₂) is the direct source of its most valuable properties: unparalleled thermal stability and chemical resistance.

The Journey from Sand to High-Performance Tube
Understanding the composition of a quartz tube requires looking at how it's made. The material isn't simply mined and drilled; it's an engineered glass with a very specific structure.
The Raw Material: Silicon Dioxide (SiO₂)
The journey begins with exceptionally pure silica sand or cultured quartz crystals. This raw material, chemically known as silicon dioxide, is the fundamental building block for the final product.
The quality of the final tube is entirely dependent on the purity of this starting material. Any contaminants, even at the parts-per-million level, can significantly degrade thermal, optical, and chemical performance.
The Fusing Process: Creating an Amorphous Glass
The raw silica is heated to extremely high temperatures (around 2000°C or 3632°F) until it melts. It is then drawn and formed into a tube and cooled rapidly.
This process "fuses" the SiO₂ molecules into a random, non-crystalline network. This amorphous or "vitreous" state is what defines it as a glass, not a crystal, and is the key to its low thermal expansion.
Fused Quartz vs. Fused Silica: A Note on Purity
While often used interchangeably, there is a technical distinction. Fused quartz is typically made by melting natural quartz crystals, while fused silica is produced synthetically from chemical precursors like silicon tetrachloride (SiCl₄).
Synthetic fused silica generally has a higher purity and better optical transmission, especially in the deep UV spectrum, making it the material of choice for applications like semiconductor manufacturing.
Why Extreme Purity Matters
The near-total absence of other elements in a quartz tube's structure is what gives it its most sought-after properties. There are simply no weak links in the chemical chain.
Exceptional Thermal Shock Resistance
Because the fused SiO₂ structure is so uniform, it has an extremely low coefficient of thermal expansion (CTE). This means it expands and contracts very little when heated or cooled.
You can heat a quartz tube to over 1000°C and plunge it into cold water without it cracking—a feat that would shatter nearly any other ceramic or glass.
High-Temperature Stability
Quartz tubes have a very high softening point (around 1650°C) and can be used continuously in applications up to approximately 1100°C. The strong silicon-oxygen bonds resist breaking down even under extreme heat.
Superior Chemical Inertness
Being composed almost entirely of SiO₂, quartz is highly resistant to attack from water, salts, and nearly all acids. This makes it an ideal container for high-purity chemical reactions where leaching from the container wall cannot be tolerated.
Understanding the Trade-offs
No material is perfect. Acknowledging the limitations of quartz is critical for successful implementation and safety.
Inherent Brittleness
Like any glass, a quartz tube is brittle. It has excellent compressive strength but is vulnerable to mechanical shock or impact. Careful handling is always required to prevent fractures.
Vulnerability to Specific Chemicals
While broadly inert, quartz will be rapidly etched and destroyed by hydrofluoric acid (HF). It is also attacked by hot phosphoric acid and strong alkaline solutions (like NaOH or KOH), especially at elevated temperatures.
Risk of Devitrification
When held at high temperatures (typically above 1100°C) for extended periods, the amorphous glass structure can slowly revert to its crystalline form (cristobalite). This process, known as devitrification, makes the quartz opaque and much more fragile, eventually leading to failure.
Making the Right Choice for Your Application
Selecting the right material requires matching its properties to your primary goal.
- If your primary focus is high-temperature processing (furnaces): The thermal stability of quartz is ideal, but be mindful of the long-term risk of devitrification if operating continuously above 1100°C.
- If your primary focus is high-purity chemistry (semiconductors): The extreme purity and chemical inertness are your greatest assets, but you must rigorously ensure your process is free of hydrofluoric acid and hot alkaline solutions.
- If your primary focus is UV optics (sterilization, curing): The excellent optical transmission of synthetic fused silica is unmatched, providing maximum efficiency for applications requiring UV light.
Ultimately, the simple and pure composition of a quartz tube is the direct source of its extraordinary capabilities in the most demanding applications.
Summary Table:
| Property | Key Characteristic |
|---|---|
| Primary Composition | High-purity Silicon Dioxide (SiO₂), >99.9% |
| Material Structure | Amorphous (non-crystalline) glass |
| Key Advantage | Unparalleled thermal shock resistance and chemical inertness |
| Thermal Limit | Continuous use up to ~1100°C |
| Main Vulnerability | Attacked by hydrofluoric acid (HF) and strong alkalis |
Ready to leverage the superior properties of quartz tubes in your laboratory? KINTEK specializes in high-purity lab equipment and consumables, including quartz tubes designed for demanding thermal and chemical processes. Our products ensure the reliability and purity your research or production requires. Contact our experts today to find the perfect quartz solution for your application!
Visual Guide
Related Products
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- Laboratory High Pressure Vacuum Tube Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
People Also Ask
- What are the low cost catalysts for pyrolysis? Optimize Your Pyrolysis Process with Affordable Catalysts
- What is the role of tube furnaces and TGA in biomass torrefaction? Optimize Your Fuel Research Parameters
- How hot does a quartz test tube get? Unlock Superior Heat Resistance for Your Lab
- How does the temperature control of a tube furnace affect the quality of graphene? Master the Optimal Thermal Window
- Why is a quartz tube furnace utilized in the thermal oxidation of MnCr2O4 coatings? Unlock Precise Selective Oxidation
- What are the advantages of using a Hastelloy (HC-276) tubular reactor for studying FeS deposition mechanisms?
- How does a laboratory tube furnace contribute to the synthesis of RuO2 catalysts? Master Thermal Precision.
- What are the primary uses of a muffle or tube furnace for LATP? Optimize Your Solid-State Electrolyte Preparation



















