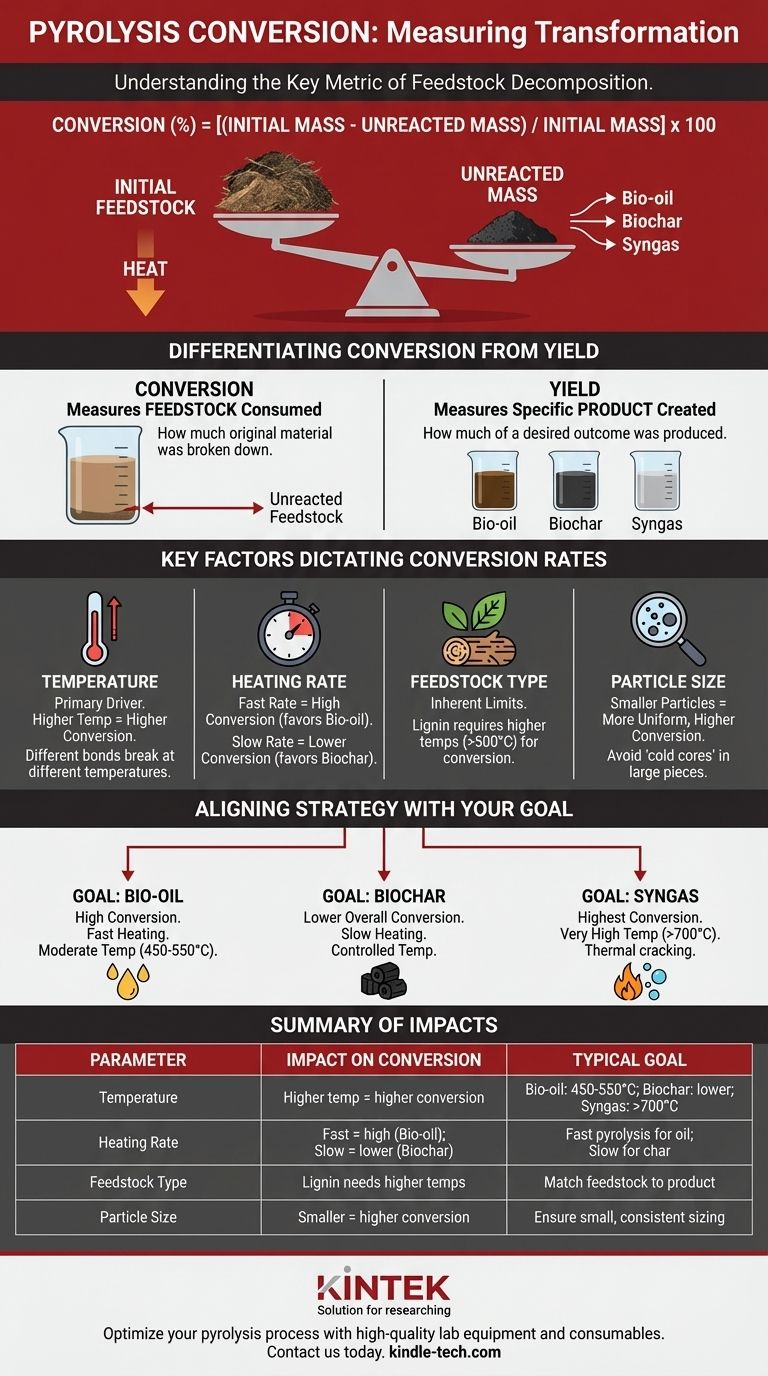
In pyrolysis, conversion is a measure of transformation. It is the key metric used to quantify how much of a raw starting material, known as feedstock, has been successfully decomposed into new products. In simple terms, it answers the question: "What percentage of the original mass was broken down by the heat?"
The central takeaway is that pyrolysis conversion measures the disappearance of the initial feedstock, not the creation of a specific product. While high conversion is often desired, the ultimate goal is not conversion for its own sake, but manipulating the process to create the most valuable mix of products—bio-oil, biochar, or syngas.

How Pyrolysis Conversion is Defined and Measured
Understanding conversion begins with a simple mass balance. It's about tracking what you start with versus what remains of that original material at the end of the process.
The Fundamental Calculation
Conversion is expressed as a percentage and calculated based on the mass of the feedstock.
The most common formula is: Conversion (%) = [(Initial Mass - Unreacted Mass) / Initial Mass] x 100
Here, "Unreacted Mass" refers to the portion of the original feedstock that did not break down and remains in its initial chemical form.
Differentiating Conversion from Yield
It is critical not to confuse conversion with yield. They measure two different things.
- Conversion measures how much of the reactant (feedstock) was consumed.
- Yield measures how much of a specific product (e.g., bio-oil) was created.
A process can have very high conversion (95% of the feedstock is gone) but a low yield of the desired product if most of it turned into something else, like gas or char.
Practical Measurement Challenges
In a real-world reactor, accurately measuring the "unreacted mass" can be difficult. It often gets mixed in with the newly formed biochar.
This requires careful post-processing and analytical techniques to distinguish between the newly created carbon structure of char and any leftover, unconverted feedstock.
Key Factors That Dictate Conversion Rates
Pyrolysis conversion is not a fixed number. It is a dynamic outcome influenced by several critical process parameters and the nature of the feedstock itself.
The Decisive Role of Temperature
Temperature is the primary driver of pyrolysis. Different chemical bonds break at different temperatures.
Higher final temperatures generally lead to higher conversion rates, as more energy is available to break down even the most resilient compounds in the feedstock, such as lignin.
The Impact of Heating Rate
How quickly the feedstock reaches the target temperature dramatically affects the outcome.
Fast pyrolysis, characterized by very high heating rates, pushes for rapid and high conversion to maximize the production of vapors that can be condensed into bio-oil.
Slow pyrolysis, with its low heating rates, results in a more gradual conversion, typically favoring a higher production of stable biochar.
The Inherent Limits of Feedstock
The chemical makeup of the feedstock sets the ultimate potential for conversion.
Materials like wood and agricultural waste are composed of cellulose, hemicellulose, and lignin. Hemicellulose decomposes at lower temperatures (220-315°C), while lignin is far more robust and requires higher temperatures (>500°C) for complete conversion.
The Influence of Particle Size
Heat transfer is key. Smaller feedstock particles heat up more quickly and uniformly, leading to a more complete and efficient conversion.
Large wood chips or pellets can suffer from "cold cores," where the interior does not reach the target temperature, leaving a significant portion of the material unreacted.
Understanding the Trade-offs: Conversion vs. Selectivity
Maximizing conversion is not always the primary objective. A skilled operator understands that the true goal is optimizing for a specific, valuable product, which often involves a trade-off with the overall conversion rate.
The Pitfall of "Conversion at All Costs"
Pushing for the highest possible conversion by using extremely high temperatures can be counterproductive.
While it ensures all feedstock is broken down, it may also cause secondary cracking of valuable long-chain molecules in bio-oil, converting them into less valuable non-condensable gases like methane and carbon monoxide.
Slow Pyrolysis: Lower Conversion for Higher Char Value
When producing biochar for agricultural or filtration purposes, the goal is to create a stable, porous carbon structure.
This process intentionally limits the complete conversion of the feedstock, using slower heating and lower peak temperatures to ensure a high yield of solid char rather than liquids and gases.
Fast Pyrolysis: High Conversion for High Bio-oil Yield
Conversely, for biofuel production, the objective is to maximize the liquid bio-oil yield.
This requires rapid heating to high temperatures to quickly convert the solid biomass into condensable vapors, deliberately minimizing the amount of char left behind. The goal is high conversion into a specific product phase.
Aligning Conversion Strategy with Your Goal
Optimizing pyrolysis conversion requires aligning your process parameters with your desired outcome. The ideal conversion rate is entirely dependent on the product you intend to create.
- If your primary focus is maximizing bio-oil production: Aim for high conversion rates using fast heating and moderate temperatures (450-550°C) to favor the formation of condensable vapors.
- If your primary focus is producing high-quality biochar: Target a lower overall mass conversion, using slow pyrolysis at controlled temperatures to ensure stable carbon structures remain as the primary product.
- If your primary focus is generating syngas: Push for the highest possible conversion at much higher temperatures (>700°C) to thermally crack all tars and chars into non-condensable gases.
Ultimately, viewing conversion as a controllable variable is the key to mastering the pyrolysis process and transforming raw materials into valuable end products.
Summary Table:
| Parameter | Impact on Conversion | Typical Goal |
|---|---|---|
| Temperature | Higher temp = higher conversion | Bio-oil: 450-550°C; Biochar: lower; Syngas: >700°C |
| Heating Rate | Fast = high conversion (bio-oil); Slow = lower conversion (biochar) | Fast pyrolysis for oil; Slow for char |
| Feedstock Type | Lignin needs higher temps for full conversion | Match feedstock to desired product |
| Particle Size | Smaller particles = more uniform, higher conversion | Ensure small, consistent sizing |
Ready to optimize your pyrolysis process for the perfect balance of conversion and product yield? At KINTEK, we specialize in providing high-quality lab equipment and consumables tailored to your pyrolysis research and development needs. Whether you're focusing on bio-oil, biochar, or syngas production, our experts can help you select the right tools to achieve precise temperature control, uniform heating, and reliable results. Contact us today to discuss how we can support your laboratory's success in converting raw materials into valuable products.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- 1700℃ Muffle Oven Furnace for Laboratory
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
People Also Ask
- What is a disadvantage of biomass energy? The Hidden Environmental and Economic Costs
- What are the different types of pyrolysis machines? Choose the Right System for Your Output
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas
- What are the reactions involved in pyrolysis of biomass? Unlock the Chemistry for Tailored Bio-Products



















