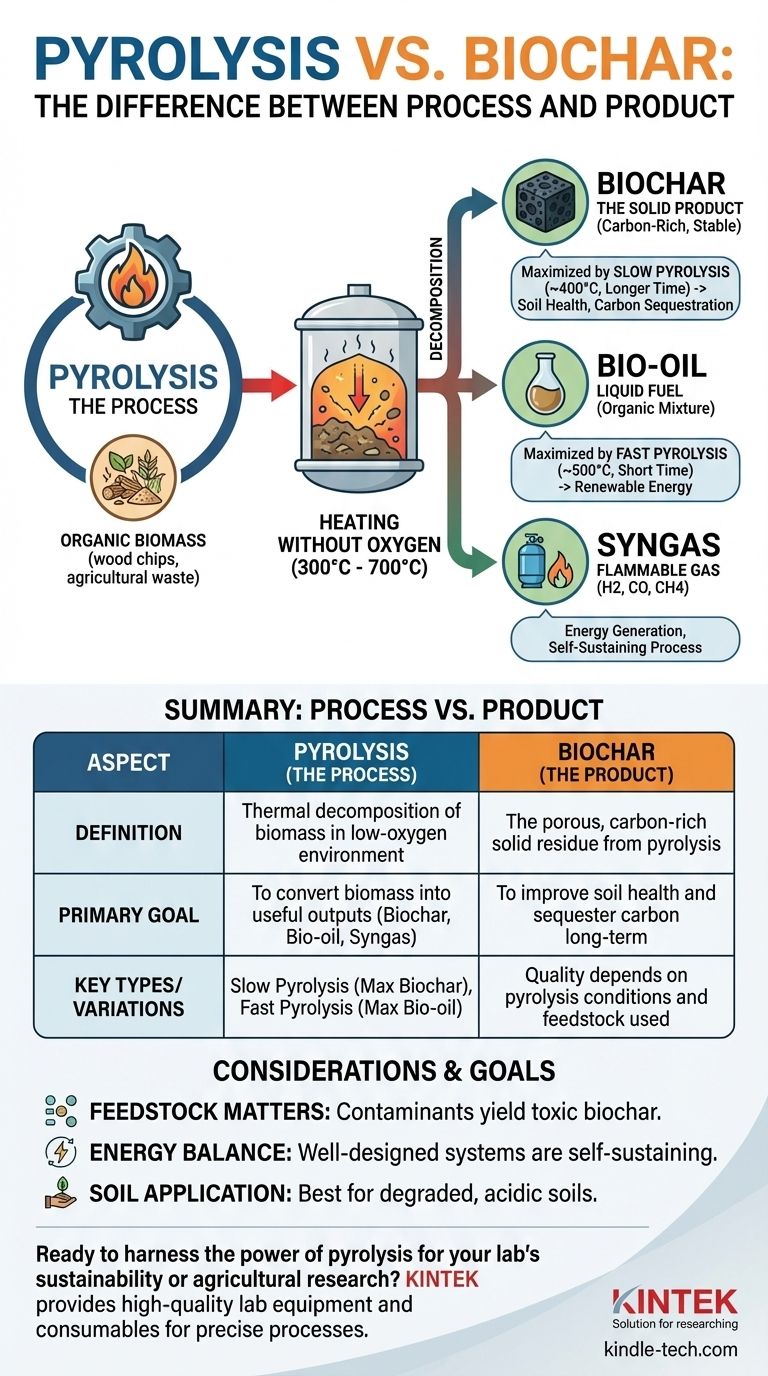
At its core, the difference is that pyrolysis is a process, while biochar is a product. Pyrolysis is the specific thermochemical method of heating organic material, such as wood or agricultural waste, in a low-oxygen environment. Biochar is the stable, carbon-rich solid that remains after that process is complete.
To put it simply, you cannot have biochar without pyrolysis. Pyrolysis is the manufacturing technique; biochar is one of its primary outputs, alongside bio-oil and syngas. Understanding this relationship is key to evaluating its role in sustainability and agriculture.

What is Pyrolysis? The Engine of Transformation
Pyrolysis is a method of thermal decomposition. It is fundamentally different from burning (combustion) because the lack of oxygen prevents the material from igniting and turning completely to ash.
The Core Principle: Heating Without Oxygen
The process involves heating biomass to temperatures typically between 300°C and 700°C in a closed container or reactor. Without sufficient oxygen, the complex molecules within the biomass break down into simpler, more stable components rather than combusting.
The Key Inputs: Organic Biomass
Virtually any organic material can be used as a feedstock for pyrolysis. This includes wood chips, crop residues (like corn stover), nut shells, manure, and even biosolids from wastewater treatment. The choice of feedstock is critical as it directly influences the final products.
The Three Primary Outputs
Pyrolysis does not just create a solid. It fractionates the biomass into three distinct outputs:
- Biochar: The solid, carbon-rich charcoal-like material.
- Bio-oil (Pyrolysis Oil): A liquid mixture of water and organic compounds.
- Syngas (Synthesis Gas): A mixture of flammable gases like hydrogen, carbon monoxide, and methane.
What is Biochar? The Stable Carbon Product
Biochar is the solid co-product of pyrolysis, distinguished by its high carbon content and remarkable stability. It is essentially a specialized form of charcoal designed for environmental and agricultural applications.
The Defining Characteristic: A Porous, Carbon-Rich Solid
Biochar's structure is incredibly porous, creating a vast internal surface area. This structure allows it to hold water and nutrients, making it an effective soil amendment. Its carbon is in a stable, aromatic form that resists decomposition for hundreds or even thousands of years.
Its Primary Purpose: Soil Health and Carbon Sequestration
The main driver for producing biochar is its ability to improve soil health by increasing water retention, nutrient availability, and microbial activity. By locking carbon into a stable solid and burying it in the soil, biochar production is also a powerful method for long-term carbon sequestration.
The Critical Link: How Pyrolysis Conditions Define the Product
The relationship between the process (pyrolysis) and the product (biochar) is controlled by the process conditions. By tuning the temperature and duration of pyrolysis, operators can choose to maximize the yield of one output over the others.
Slow Pyrolysis: Maximizing Biochar Yield
To produce the most biochar, a process called slow pyrolysis is used. This involves lower temperatures (around 400°C) and a longer processing time. Under these conditions, biochar yields can be as high as 30% of the initial dry feedstock weight, as more of the carbon remains in a solid form.
Fast Pyrolysis: Maximizing Bio-oil Yield
Conversely, if the goal is to produce liquid fuel, fast pyrolysis is used. This process uses higher temperatures (around 500°C) and extremely short heating times (often less than two seconds). This cracks the biomass molecules into vapors that are then rapidly cooled to form bio-oil, with biochar being a less abundant secondary product.
Understanding the Trade-offs and Considerations
While a powerful tool, the pyrolysis-biochar system is not a magic bullet. Objective analysis requires acknowledging its limitations.
The Feedstock Dilemma
The quality of the biochar is entirely dependent on the input feedstock. Using biomass contaminated with heavy metals, plastics, or other pollutants will result in a toxic biochar that can harm soil and leach contaminants into groundwater.
Process Energy Balance
Pyrolysis requires a significant energy input to reach operating temperatures. A well-designed system will use the syngas it produces as fuel to become self-sustaining, but poorly designed systems can be net energy consumers.
Application is Not Universal
Biochar is not beneficial for all soil types. Its greatest positive impact is seen in degraded, acidic, or sandy soils with low organic matter. In already fertile, high-carbon soils, its benefits may be minimal or non-existent.
Making the Right Choice for Your Goal
Your interest in these terms likely stems from a specific goal. Use this framework to clarify your focus.
- If your primary focus is carbon sequestration and soil improvement: You are most interested in the biochar product itself, specifically biochar created via slow pyrolysis to ensure maximum stability and yield.
- If your primary focus is renewable energy production: You are most interested in the pyrolysis process, particularly fast pyrolysis, which maximizes the generation of combustible bio-oil and syngas.
- If your primary focus is sustainable waste management: You must evaluate the entire pyrolysis system, analyzing your feedstock for contaminants and finding value for all three outputs—biochar, bio-oil, and syngas—to create an economically and environmentally viable model.
Understanding that pyrolysis is the tool and biochar is the result is the first step toward effectively harnessing this technology for agricultural, environmental, or energy applications.
Summary Table:
| Aspect | Pyrolysis (The Process) | Biochar (The Product) |
|---|---|---|
| Definition | Thermal decomposition of biomass in a low-oxygen environment. | The porous, carbon-rich solid residue from pyrolysis. |
| Primary Goal | To convert biomass into useful outputs: biochar, bio-oil, and syngas. | To improve soil health and sequester carbon long-term. |
| Key Types | Slow Pyrolysis (maximizes biochar), Fast Pyrolysis (maximizes bio-oil). | Quality and properties depend on the pyrolysis conditions and feedstock used. |
Ready to harness the power of pyrolysis for your lab's sustainability or agricultural research? KINTEK specializes in high-quality lab equipment and consumables, providing the reliable tools you need for precise pyrolysis processes and biochar analysis. Whether you're developing new soil amendments or exploring renewable energy, our solutions support your innovation. Contact our experts today to find the perfect equipment for your specific application!
Visual Guide
Related Products
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- Graphite Vacuum Continuous Graphitization Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- Vertical Laboratory Tube Furnace
People Also Ask
- Why use quartz tubes and vacuum sealing for sulfide solid-state electrolytes? Ensure Purity & Stoichiometry
- What happens when quartz is heated? A Guide to Its Critical Phase Transitions and Uses
- What is the function of quartz tubes and vacuum sealing systems? Secure Your High-Purity Solid Solution Synthesis
- Why are quartz tubes preferred for chromium powder combustion? Superior Heat Resistance & Optical Clarity
- What role does a quartz tube furnace play in hBN synthesis? Optimize Your Chemical Vapor Deposition Results



















