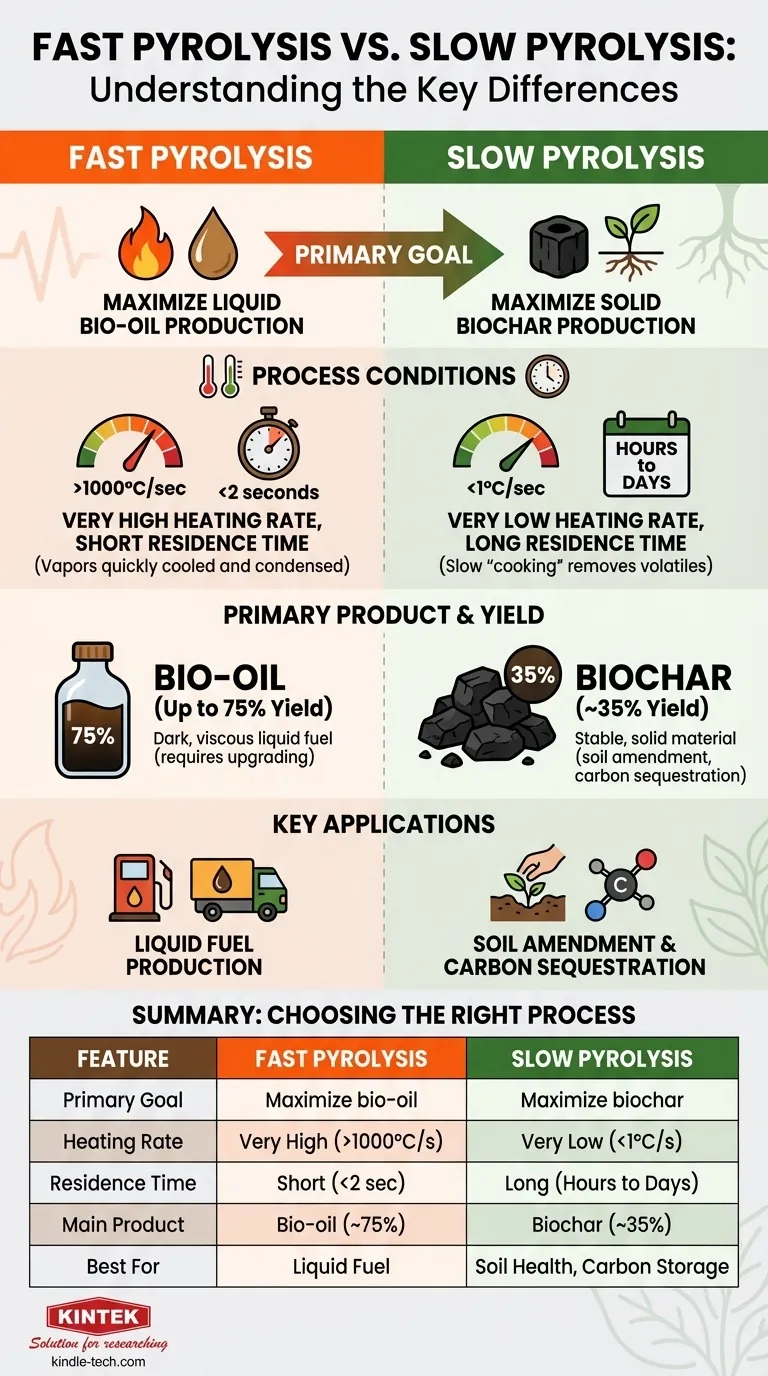
The primary difference between fast and slow pyrolysis lies in the process conditions, which are intentionally manipulated to favor the production of either liquid bio-oil or solid biochar. Fast pyrolysis uses extremely high heating rates and short reaction times to maximize the liquid yield, whereas slow pyrolysis uses low heating rates over long periods to maximize the solid, carbon-rich char.
The choice between fast and slow pyrolysis is a strategic decision based on your desired end product. Fast pyrolysis is engineered to convert biomass into a liquid fuel (bio-oil), while slow pyrolysis is optimized to produce a stable, solid material (biochar).

Fast Pyrolysis: Maximizing Liquid Bio-oil
Fast pyrolysis is a rapid thermal decomposition process designed to break down biomass into vapors, which are then quickly cooled and condensed into a liquid.
### The Critical Process Conditions
The process is defined by an extremely high heating rate (over 1000°C per second) and a very short vapor residence time (typically less than two seconds). The goal is to heat the biomass so quickly that it vaporizes before it can undergo the secondary reactions that form solid char.
### The Primary Product: Bio-oil
The main output, often representing up to 75% of the product yield by weight, is bio-oil. This dark, viscous liquid is a complex mixture of oxygenated organic compounds. While it is a form of liquid fuel, it is acidic and unstable, often requiring significant upgrading before it can be used as a replacement for conventional fuels.
### Key Advantages
Fast pyrolysis is a relatively quick and efficient method for converting the entirety of a biomass feedstock into a transportable fuel product. As noted in research, facilities can be built on a smaller, mobile scale to process biomass near its source, reducing transportation costs for the raw material.
Slow Pyrolysis: Optimizing for Solid Biochar
In contrast, slow pyrolysis is a much more deliberate process. It has been used for centuries to produce charcoal, and its modern application focuses on creating a stable, carbon-rich product called biochar.
### The Critical Process Conditions
Slow pyrolysis is characterized by a very low heating rate (less than 1°C per second) and a very long residence time, which can range from several hours to even days. This slow "cooking" process systematically drives off volatile compounds, leaving behind a fixed carbon structure.
### The Primary Product: Biochar
The primary output is biochar, a solid material that can make up around 35% of the product yield. Biochar is highly valued for its ability to improve soil health, retain water, and sequester carbon in the ground for long periods. The process also produces some bio-oil and syngas, but in much smaller quantities than fast pyrolysis.
Understanding the Trade-offs
Neither process is inherently superior; the optimal choice depends entirely on the intended application and the available resources.
### The Challenge of Bio-oil
While fast pyrolysis is efficient at creating liquid fuel, bio-oil is not a drop-in replacement for gasoline or diesel. It must be upgraded through processes like hydrotreating to remove oxygen and improve its stability, which adds complexity and cost to the overall fuel production chain.
### The Variable Nature of Biochar
The quality and properties of biochar from slow pyrolysis are highly dependent on the specific feedstock and process conditions. This variability can make it difficult to define a consistent market value for the product, as its effectiveness as a soil amendment can change significantly from batch to batch.
### Reactor Technology
The choice of process often dictates the type of reactor used. Fast pyrolysis requires advanced reactors like fluidized-bed or ablative reactors that can achieve rapid heat transfer. Slow pyrolysis can be accomplished with simpler, less dynamic systems like rotary kilns or fixed-bed reactors.
Making the Right Choice for Your Goal
To select the appropriate method, you must first define your primary objective.
- If your primary focus is producing a liquid fuel for energy: Fast pyrolysis is the correct pathway, as it is specifically designed to maximize the yield of bio-oil from biomass.
- If your primary focus is carbon sequestration or soil amendment: Slow pyrolysis is the superior choice because it is optimized to create stable, high-carbon biochar.
Ultimately, understanding the fundamental goals of each process empowers you to select the technology that aligns with your specific outcome.
Summary Table:
| Feature | Fast Pyrolysis | Slow Pyrolysis |
|---|---|---|
| Primary Goal | Maximize liquid bio-oil production | Maximize solid biochar production |
| Heating Rate | Very high (>1000°C/sec) | Very low (<1°C/sec) |
| Residence Time | Short (<2 seconds) | Long (hours to days) |
| Main Product | Bio-oil (up to 75% yield) | Biochar (~35% yield) |
| Best For | Liquid fuel production | Soil amendment, carbon sequestration |
Ready to select the right pyrolysis process for your biomass conversion needs? At KINTEK, we specialize in providing high-quality lab equipment and consumables for all your pyrolysis research and development. Whether you're optimizing for bio-oil or biochar, our reactors and analytical tools deliver the precision and reliability you need. Contact our experts today to discuss how we can support your laboratory's specific pyrolysis applications and help you achieve your biofuel goals.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
People Also Ask
- What is the optimum temperature for pyrolysis? Target Your Desired Biochar, Bio-oil, or Syngas
- What is the difference between calcination and roasting? A Guide to Thermal Treatment Processes
- What are the different temperatures of pyrolysis? A Guide to Optimizing Biochar, Bio-Oil, and Syngas Production
- What are the different types of reactors in plastic pyrolysis? Choose the Right System for Your Waste
- What is the difference between fast and slow biomass pyrolysis? Optimize Your Biofuel or Biochar Production
- What is fast pyrolysis of wood? A Rapid Process to Maximize Bio-Oil Yield
- What is the difference between batch and continuous pyrolysis? Choose the Right System for Your Scale
- What is calcination a burning process? Discover the Key Differences in Thermal Processing













