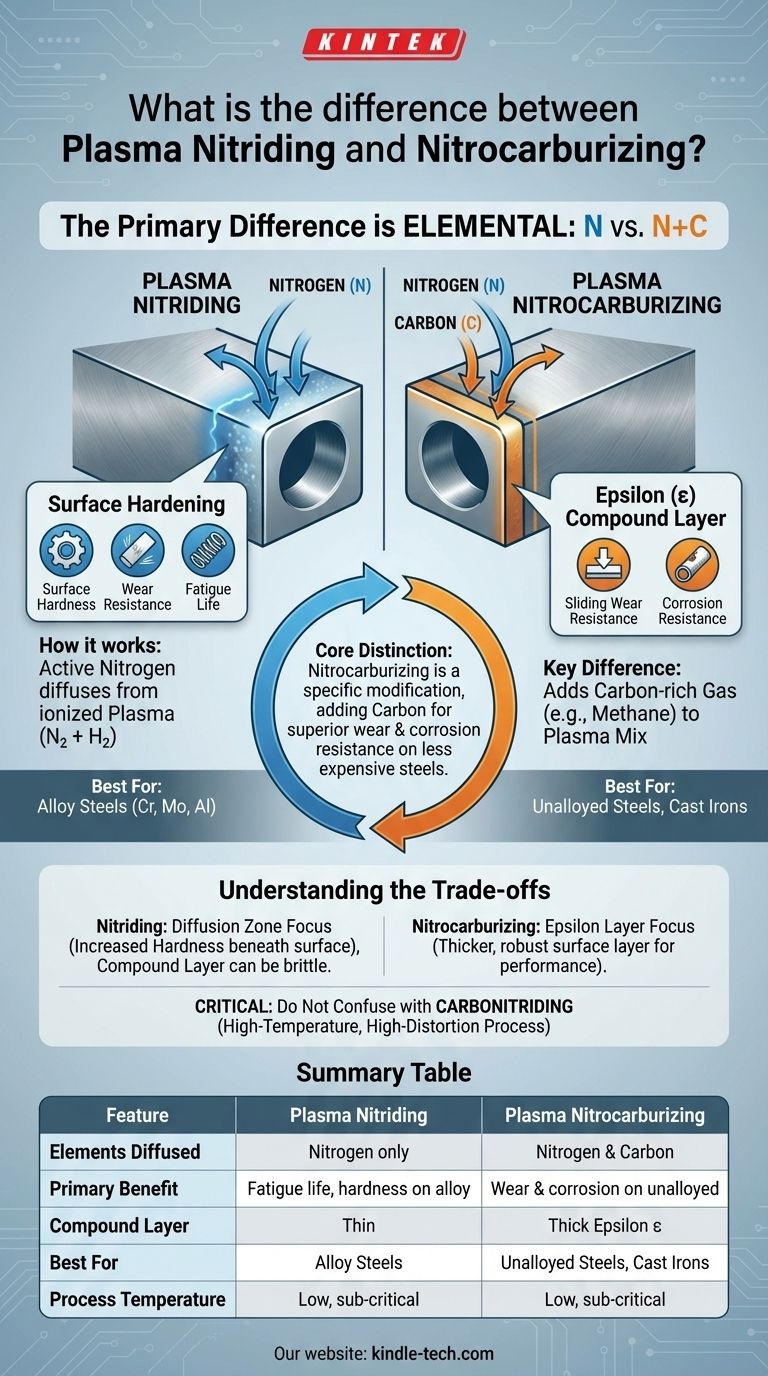
The primary difference is elemental. Plasma nitriding is a surface hardening process that diffuses only nitrogen into a metal's surface. Plasma nitrocarburizing is a variation of this process that diffuses both nitrogen and a small amount of carbon into the surface, creating a distinct compound layer with unique properties.
The core distinction isn't about two competing processes, but about one being a specific modification of the other. Nitrocarburizing adds carbon to the nitriding process to achieve superior wear and corrosion resistance, particularly on less expensive, unalloyed steels.
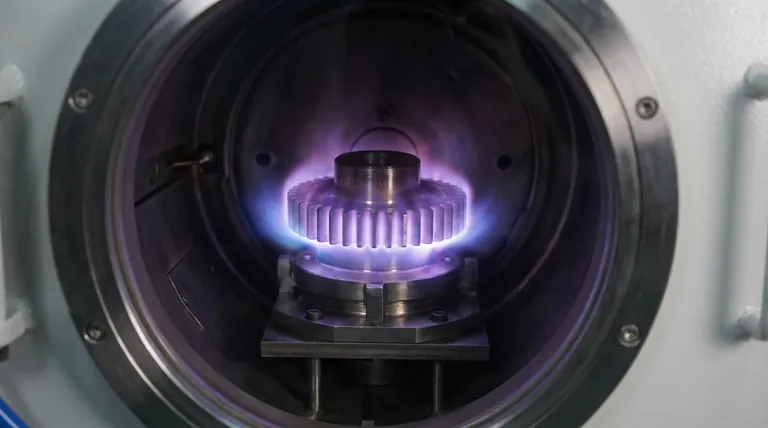
The Foundation: Understanding Plasma Nitriding
The Goal of Surface Hardening
Nitriding is a thermochemical case-hardening process. Its purpose is to significantly improve the surface properties of a metal part without altering the core material's toughness.
The primary benefits are increased surface hardness, enhanced wear resistance, and a major improvement in fatigue life.
How Nitriding Works
The process involves heating a ferrous metal component to a controlled, sub-critical temperature (below the point where the core structure changes). It is then exposed to active nitrogen.
This active nitrogen diffuses into the surface of the steel, forming hard metallic nitrides with the iron and other alloying elements present.
The "Plasma" Method
"Plasma" simply describes the method used to create the active nitrogen. In this process, a mixture of nitrogen and hydrogen gas is excited by a high-voltage electrical field within a vacuum.
This creates an ionized gas, or plasma, which efficiently delivers nitrogen ions to the component's surface for diffusion.
The Variation: Introducing Nitrocarburizing
The Key Difference: Adding Carbon
Plasma nitrocarburizing begins with the same nitrogen-hydrogen gas mixture as plasma nitriding. However, a small amount of a carbon-rich gas, like methane or carbon dioxide (typically 1-3%), is added to the mix.
This addition allows for the co-diffusion of both nitrogen and carbon into the steel's surface.
The Result: The Epsilon (ε) Compound Layer
The introduction of carbon promotes the formation of a specific surface layer known as the epsilon (ε) compound layer (Fe₂-₃CₓNᵧ).
This layer is particularly dense and stable, offering excellent resistance to sliding wear, scuffing, and corrosion.
When to Use Nitrocarburizing
This process is especially effective and commonly used for materials that do not contain strong nitride-forming alloy elements.
It is a preferred treatment for unalloyed (plain carbon) steels and cast irons, where standard nitriding would be less effective.
Understanding the Trade-offs
Compound Layer vs. Diffusion Zone
In standard nitriding, the primary benefit often comes from the "diffusion zone" beneath the surface where hardness is increased. The thin compound layer on the very top can sometimes be brittle.
Nitrocarburizing is specifically designed to create a thicker, more robust, and more ductile compound layer. The goal is this epsilon layer, which provides the primary performance enhancement.
Material Suitability
Standard nitriding is most effective on alloy steels containing elements like chromium, molybdenum, and aluminum, which are strong nitride formers.
Nitrocarburizing broadens the applicability of nitriding to cheaper materials, providing a hard, wear-resistant case on plain carbon steels.
A Common Point of Confusion: Carbonitriding
It is critical not to confuse nitrocarburizing with carbonitriding. Carbonitriding is a completely different, high-temperature process (typically 1450°F - 1550°F) that adds both carbon and nitrogen but functions more like carburizing.
Nitrocarburizing, like nitriding, is a low-temperature, sub-critical process that results in far less distortion and is fundamentally different in its metallurgical outcome.
Making the Right Choice for Your Component
Choosing the correct process depends entirely on the base material and the desired performance outcome.
- If your primary focus is increasing fatigue life and surface hardness on an alloy steel: Standard plasma nitriding is the most effective choice.
- If your primary focus is creating excellent wear and corrosion resistance on an unalloyed steel or cast iron: Plasma nitrocarburizing is the definitive solution.
- If you need a thin, hard case on a low-carbon steel and can tolerate higher temperatures and some distortion: You should investigate the separate process of carbonitriding.
Understanding this distinction empowers you to select the precise surface treatment for optimal component performance and cost-effectiveness.
Summary Table:
| Feature | Plasma Nitriding | Plasma Nitrocarburizing |
|---|---|---|
| Elements Diffused | Nitrogen only | Nitrogen and Carbon |
| Primary Benefit | Fatigue life, surface hardness on alloy steels | Wear & corrosion resistance on unalloyed steels/cast iron |
| Compound Layer | Thin, sometimes brittle | Thick, robust epsilon (ε) layer |
| Best For | Alloy steels (Cr, Mo, Al) | Unalloyed / plain carbon steels, cast irons |
| Process Temperature | Low-temperature, sub-critical | Low-temperature, sub-critical |
Need help selecting the optimal surface treatment for your components? KINTEK specializes in advanced thermal processing solutions, including plasma nitriding and nitrocarburizing systems. Our expertise ensures you achieve the precise surface hardness, wear resistance, and fatigue life your laboratory or manufacturing process demands. Contact our experts today to discuss your specific material and performance requirements!
Visual Guide
Related Products
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
- Molybdenum Vacuum Heat Treat Furnace
- Vacuum Heat Treat Sintering Brazing Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Vertical Laboratory Tube Furnace
People Also Ask
- What is the function of a high-temperature solution furnace in PWHT? Restore Alloy 800H Joint Integrity
- Why is a high-precision tempering furnace required for PM-HIP joints? Ensure Toughness in SA508 Steel
- What is the role of a vacuum drying oven in pre-treating granite? Ensure Data Integrity with Low-Temp Precision
- Why is a high-vacuum pumping system necessary during the gas-phase hydrogenation of Zr1Nb alloys? Ensure Material Purity
- How does the precision temperature control of an electric heating furnace influence zinc borate? Master Microstructure
- What is the primary purpose of any heat treatment operation? To Tailor Material Properties for Performance
- How is vacuum packing different from normal packing? A Guide to Industrial Vacuum Hardening
- What is the difference between sintering and fusing? Master the Key Thermal Processes for Your Materials



















