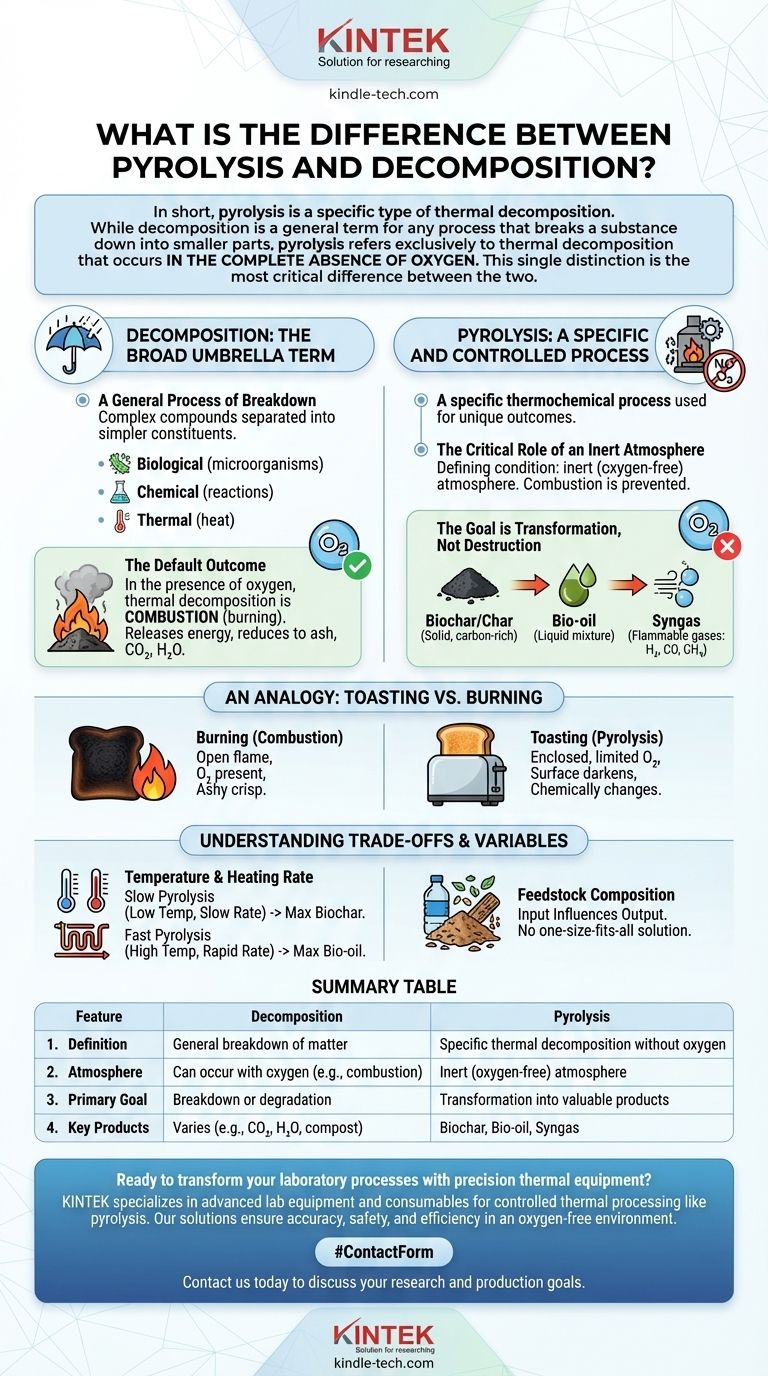
In short, pyrolysis is a specific type of thermal decomposition. While decomposition is a general term for any process that breaks a substance down into smaller parts, pyrolysis refers exclusively to thermal decomposition that occurs in the complete absence of oxygen. This single distinction is the most critical difference between the two.
Think of decomposition as the broad category of "breaking down." Pyrolysis is a precise, engineered process within that category, defined by using high heat without oxygen to transform material into valuable new products, not simply to let it degrade or burn into ash.
Decomposition: The Broad Umbrella Term
Decomposition is a fundamental concept across biology, chemistry, and environmental science. It is not one specific process but a general classification for the breakdown of complex matter.
A General Process of Breakdown
At its core, decomposition means a complex compound or substance is separated into simpler constituents. This can happen through many different mechanisms, not just heat.
Multiple Pathways Exist
Decomposition can be biological, driven by microorganisms like bacteria and fungi (e.g., a rotting log in the forest). It can be chemical, caused by reactions with other substances. And it can be thermal, where heat provides the energy to break chemical bonds.
The Default Outcome
In most natural settings, decomposition occurs in the presence of oxygen. Thermal decomposition with oxygen is simply combustion (burning), which releases energy and reduces the material primarily to ash, carbon dioxide, and water.
Pyrolysis: A Specific and Controlled Process
Pyrolysis is not a natural occurrence in the same way as general decay. It is a specific thermochemical process used in engineering and industrial applications for its unique outcomes.
The Critical Role of an Inert Atmosphere
The defining condition of pyrolysis is an inert (oxygen-free) atmosphere. By removing oxygen, combustion is prevented. The material cannot burn.
Instead of being destroyed and releasing its chemical energy as heat, the feedstock's chemical bonds are broken by the high temperatures, reforming into new, often valuable, molecules.
The Goal is Transformation, Not Destruction
Because burning is avoided, pyrolysis transforms the original material into three distinct product types:
- Biochar/Char: A solid, carbon-rich residue.
- Bio-oil/Pyrolysis Oil: A liquid mixture of various organic compounds.
- Syngas: A mixture of flammable gases (like hydrogen, carbon monoxide, and methane).
An Analogy: Toasting vs. Burning
Imagine a slice of bread. If you put it over an open flame (oxygen is present), it will catch fire and burn to a black, ashy crisp. This is like combustion.
If you put that same bread in a toaster (a hot, enclosed environment with limited oxygen), it turns into toast. The surface darkens and chemically changes, but it doesn't burn to ash. This is a simplified analogy for pyrolysis, where the material is transformed by heat rather than incinerated.
Understanding the Trade-offs and Key Variables
The outcome of pyrolysis is not accidental; it is controlled by manipulating specific process parameters. Misunderstanding this is a common pitfall.
Temperature and Heating Rate
The final yields of char, oil, and gas are directly influenced by temperature.
- Slow Pyrolysis: Lower temperatures and slow heating rates maximize the production of solid biochar. This is the traditional method for making charcoal.
- Fast Pyrolysis: High temperatures and extremely rapid heating rates maximize the production of liquid bio-oil. This is a focus for producing advanced biofuels.
Feedstock Composition
The process is not magic. What you put in heavily influences what you get out. Pyrolyzing waste plastic produces a very different oil and gas composition compared to pyrolyzing wood chips or agricultural waste. There is no one-size-fits-all solution.
Making the Right Choice for Your Goal
Using these terms correctly signals a clear understanding of the underlying processes and their purpose.
- If your primary focus is environmental science or biology: Use "decomposition" as the default term for natural breakdown processes like composting or decay.
- If your primary focus is chemical engineering or materials processing: Use "pyrolysis" specifically for controlled thermal treatment in an oxygen-free environment to create char, oil, and gas.
- If you are evaluating waste-to-value technology: Clearly distinguish pyrolysis from "combustion" (burning with excess oxygen) and "gasification" (partial oxidation with limited oxygen).
Using these terms with precision demonstrates a fundamental understanding of whether a material is being destroyed, degraded, or intentionally transformed.

Summary Table:
| Feature | Decomposition | Pyrolysis |
|---|---|---|
| Definition | General breakdown of matter | Specific thermal decomposition without oxygen |
| Atmosphere | Can occur with oxygen (e.g., combustion) | Inert (oxygen-free) atmosphere |
| Primary Goal | Breakdown or degradation | Transformation into valuable products |
| Key Products | Varies (e.g., CO₂, H₂O, compost) | Biochar (solid), Bio-oil (liquid), Syngas |
Ready to transform your laboratory processes with precision thermal equipment?
KINTEK specializes in advanced lab equipment and consumables for controlled thermal processing like pyrolysis. Whether you're developing biofuels, producing biochar, or analyzing material transformation, our solutions ensure accuracy, safety, and efficiency in an oxygen-free environment.
Contact us today using the form below to discuss how our expertise can help you achieve your research and production goals. Let's turn your feedstock into valuable products together.
Visual Guide
Related Products
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Vertical Laboratory Tube Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What is the ceramic tube high temperature? From 1100°C to 1800°C, Choose the Right Material
- What is the pressure on a tube furnace? Essential Safety Limits for Your Lab
- What are the advantages of using an alumina liner in a tube furnace for biomass combustion corrosion simulations?
- What temperature is alumina activated? Unlock Optimal Porosity for Adsorption
- Why is a high-purity alumina lining required for high-temperature tube furnaces? Ensure Accurate Biomass Research



















