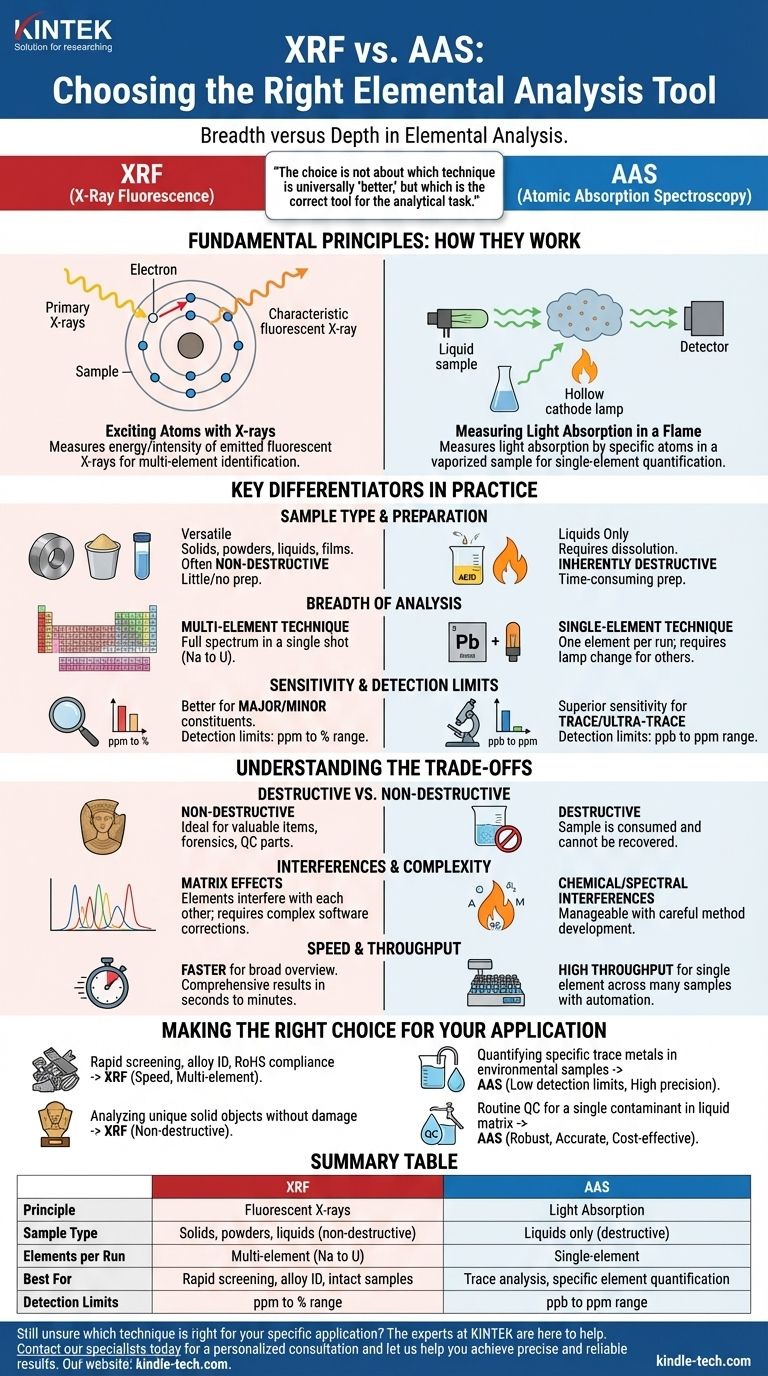
At its core, the difference between X-Ray Fluorescence (XRF) and Atomic Absorption Spectroscopy (AAS) comes down to breadth versus depth. XRF is a rapid survey technique that uses X-rays to identify and quantify a wide range of elements in a single analysis, often non-destructively. In contrast, AAS is a highly sensitive method that dissolves a sample to measure the precise concentration of one specific element at a time.
The choice is not about which technique is universally "better," but which is the correct tool for the analytical task. XRF excels at rapid, multi-element characterization of intact samples, while AAS provides high-precision, single-element measurement for trace-level concentrations.

The Fundamental Principles: How They Work
To understand their practical differences, you must first grasp their distinct operating principles. Each method interacts with a sample's atoms in a fundamentally different way.
XRF: Exciting Atoms with X-rays
X-Ray Fluorescence works by bombarding a sample with high-energy primary X-rays. This energy strikes atoms within the sample and knocks out an electron from an inner electron shell.
This creates an unstable vacancy, which is immediately filled by an electron from a higher-energy outer shell. As this electron "falls" into the lower energy state, it releases a secondary, or fluorescent, X-ray.
The energy of this fluorescent X-ray is a unique fingerprint for each element. The XRF detector measures the energies and intensities of all the fluorescent X-rays emitted, allowing it to determine which elements are present and in what amounts, all at once.
AAS: Measuring Light Absorption in a Flame
Atomic Absorption Spectroscopy operates on a completely different principle. It begins by converting the sample, which must be in a liquid form, into a fine aerosol.
This aerosol is then introduced into a high-temperature flame or a graphite furnace, a process that atomizes the sample into a cloud of free, ground-state atoms.
A special lamp, called a hollow cathode lamp, emits a beam of light at a wavelength known to be absorbed specifically by the one element you are targeting. This light passes through the atom cloud. The target atoms absorb a portion of this light, and a detector measures how much light made it through. The amount of light absorbed is directly proportional to the concentration of that single element.
Key Differentiators in Practice
The underlying principles of XRF and AAS lead to critical differences in their application, speed, and sensitivity.
Sample Type and Preparation
XRF is exceptionally versatile, capable of analyzing solids, powders, liquids, and films directly, often with little to no sample preparation. This makes it ideal for analyzing finished parts, geological cores, or metal alloys as-is.
AAS, by contrast, requires the sample to be completely dissolved into a liquid solution through acid digestion. This is an inherently destructive and often time-consuming step that requires a skilled technician.
Breadth of Analysis
This is the most significant practical distinction. XRF is a multi-element technique. A single measurement, often taking seconds to minutes, provides a full elemental spectrum, typically from sodium (Na) to uranium (U).
AAS is a single-element technique. To measure a different element, you must physically change the light source to the specific lamp for that new element and run an entirely new analysis.
Sensitivity and Detection Limits
AAS generally offers superior sensitivity and lower detection limits. It is the preferred method for trace and ultra-trace analysis, routinely measuring concentrations in the parts-per-million (ppm) and even parts-per-billion (ppb) range.
XRF is better suited for analyzing major and minor constituents. Its detection limits are typically in the low-ppm to percentage (%) range, making it less suitable for ultra-trace contaminant analysis.
Understanding the Trade-offs
Choosing between these methods involves weighing their respective strengths against their limitations. Neither is a perfect solution for every problem.
Destructive vs. Non-Destructive
The most crucial trade-off is often the sample itself. Because XRF can analyze samples in their native state, it is considered non-destructive. This is essential when analyzing valuable or irreplaceable items like archeological artifacts, forensic evidence, or critical quality control parts.
AAS is fundamentally destructive. The sample must be dissolved and vaporized, meaning it cannot be recovered after the analysis is complete.
Interferences and Complexity
Both techniques are subject to interferences that can affect accuracy. In XRF, this is known as a matrix effect, where the presence of other elements in the sample can enhance or absorb the fluorescent X-rays of the target element, requiring complex software corrections.
In AAS, interferences are primarily chemical or spectral in nature within the flame. While these require careful method development to overcome, they are often well-understood and manageable for specific applications.
Speed and Throughput
For a broad elemental understanding of a sample, XRF is significantly faster. It provides a comprehensive qualitative and quantitative overview in a single shot.
However, if you need to measure the concentration of only one specific element across hundreds of samples, an AAS system with an autosampler can offer very high throughput once the initial setup and method development are complete.
Making the Right Choice for Your Application
Your analytical goal is the only factor that matters when selecting a technique. Consider the primary question you need to answer.
- If your primary focus is rapid screening and alloy identification (e.g., scrap metal sorting, RoHS compliance): XRF is the definitive choice for its speed, multi-element capability, and ease of use.
- If your primary focus is quantifying specific trace metals in environmental samples (e.g., lead in drinking water): AAS provides the low detection limits and high precision required for regulatory compliance.
- If your primary focus is analyzing unique solid objects without damaging them (e.g., museum artifacts, jewelry): XRF's non-destructive nature makes it the only feasible option.
- If your primary focus is routine quality control for a known, single contaminant in a liquid matrix: AAS is a robust, accurate, and cost-effective workhorse for this task.
Ultimately, understanding whether your goal is a broad survey or a precise, targeted measurement is the key to selecting the correct elemental analysis tool.
Summary Table:
| Feature | XRF (X-Ray Fluorescence) | AAS (Atomic Absorption Spectroscopy) |
|---|---|---|
| Principle | Measures fluorescent X-rays from sample | Measures light absorption by atomized sample |
| Sample Type | Solids, powders, liquids (often non-destructive) | Liquids only (destructive) |
| Elements per Run | Multi-element (Na to U) | Single-element |
| Best For | Rapid screening, alloy ID, intact samples | Trace analysis, specific element quantification |
| Detection Limits | ppm to % range | ppb to ppm range |
Still unsure which technique is right for your specific application? The experts at KINTEK are here to help. We specialize in laboratory equipment and consumables, providing tailored solutions for your elemental analysis needs. Whether you require the broad screening capabilities of XRF or the precise trace detection of AAS, we can guide you to the optimal instrument for your lab's workflow, budget, and accuracy requirements.
Contact our specialists today for a personalized consultation and let us help you achieve precise and reliable results.
Visual Guide
Related Products
- Customizable XRD Sample Holders for Diverse Research Applications
- Laboratory Test Sieves and Vibratory Sieve Shaker Machine
- Metallographic Specimen Mounting Machine for Laboratory Materials and Analysis
- Three-dimensional electromagnetic sieving instrument
- Double Plate Heating Press Mold for Lab
People Also Ask
- What is the difference between XRF and XRD techniques? A Guide to Choosing the Right Analytical Tool
- What affects melting point chemistry? A Guide to Molecular Forces and Lattice Energy
- How can corrosion of the sample holder be prevented when using corrosive chemicals? Protect Your Lab's Integrity
- What are the factors that affect melting and boiling point? Unlock the Science of Phase Transitions
- How should a sample holder be handled to ensure its longevity? Protect Your Lab Investment and Data Integrity















