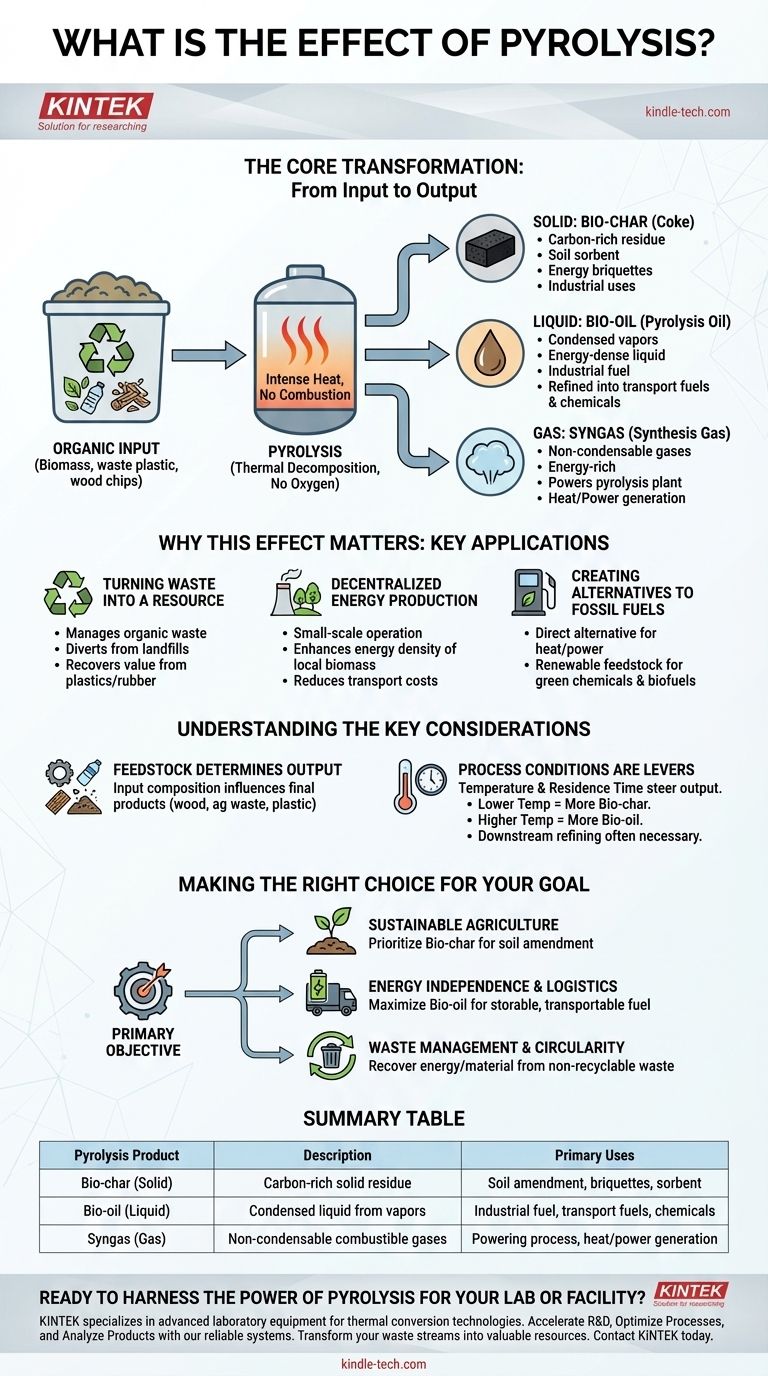
In short, the primary effect of pyrolysis is the thermal decomposition of organic material in the absence of oxygen. This process transforms a single input, like biomass or plastic waste, into three distinct and valuable outputs: a solid char, a liquid oil, and a combustible gas.
Pyrolysis should not be viewed as a disposal method, but as a versatile conversion technology. Its core effect is to unlock the chemical and energetic value trapped in low-value organic materials, turning waste streams into valuable resources like fuel, chemicals, and soil amendments.

The Core Transformation: From Input to Output
Pyrolysis fundamentally restructures organic matter by breaking down large, complex molecules into smaller, more useful ones through intense heat without combustion. This controlled process allows for the recovery of valuable products.
The Solid Product: Bio-char
The solid residue left after pyrolysis is a carbon-rich material known as bio-char or coke.
This product is not waste; it has significant applications in agriculture as a soil sorbent, in energy production as briquettes, and in industrial processes.
The Liquid Product: Bio-oil
As the organic material breaks down, volatile components are released as vapors, which are then condensed into a liquid known as pyrolysis oil or bio-oil.
This liquid is energy-dense and can be stored and transported far more easily than the original biomass. It can be used directly as an industrial fuel or further refined into higher-grade products like transportation fuels and specialty chemicals.
The Gaseous Product: Syngas
The process also generates non-condensable gases, collectively called syngas (synthesis gas).
This gas is rich in energy and is often used to power the pyrolysis plant itself, creating a self-sustaining and highly efficient thermal loop. Excess gas can be used for heat or power generation.
Why This Effect Matters: Key Applications
The ability to convert organic matter into three distinct product streams makes pyrolysis a powerful tool for solving critical environmental and economic challenges.
Turning Waste into a Resource
Pyrolysis offers a robust solution for managing organic waste from agriculture, forestry, and municipal sources.
It diverts significant volume from landfills and enables the recovery of valuable materials from waste plastics and rubber, reducing the environmental impact and the need for virgin raw materials.
Decentralized Energy Production
Unlike large, centralized power plants, pyrolysis units can be operated at a relatively small scale and in remote locations.
This capability enhances the energy density of local biomass, dramatically reducing the costs and complexities of transporting bulky raw materials. It converts a logistical problem into a localized energy solution.
Creating Alternatives to Fossil Fuels
The bio-oil and syngas produced are direct alternatives to fossil fuels for generating heat and power.
Furthermore, the bio-oil serves as a renewable feedstock for producing green chemicals and advanced biofuels, contributing to a more sustainable industrial ecosystem.
Understanding the Key Considerations
While powerful, the effects of pyrolysis are not automatic. The process must be carefully controlled to achieve desired outcomes, as the balance of solid, liquid, and gas products is highly dependent on process conditions.
Feedstock Determines Output
The specific composition of the input material—whether it's wood, agricultural waste, or plastic—directly influences the chemical makeup and quality of the final products.
Process Conditions Are Levers
Engineers can manipulate variables like temperature and residence time (how long the material stays in the reactor) to steer the output.
Slower pyrolysis at lower temperatures typically maximizes the bio-char yield, while fast pyrolysis at higher temperatures is used to maximize bio-oil production. This control is critical for targeting specific end products.
Downstream Refining is Often Necessary
While bio-oil is a valuable product, it is often acidic and unstable compared to petroleum fuels. It typically requires upgrading or refining before it can be used as a drop-in transportation fuel, adding a layer of technical complexity and cost.
Making the Right Choice for Your Goal
The optimal application of pyrolysis depends entirely on your primary objective.
- If your primary focus is sustainable agriculture or carbon sequestration: Prioritize a process that maximizes the output of stable, high-quality bio-char for soil amendment.
- If your primary focus is energy independence and logistics: Use pyrolysis to convert bulky, local biomass into dense, storable bio-oil, creating a transportable fuel source.
- If your primary focus is waste management and circularity: Apply pyrolysis to recover the energy and material value from non-recyclable plastics and organic municipal waste.
Ultimately, pyrolysis is a key enabling technology for a circular economy, transforming our concept of waste into one of opportunity.
Summary Table:
| Pyrolysis Product | Description | Primary Uses |
|---|---|---|
| Bio-char (Solid) | Carbon-rich solid residue | Soil amendment, industrial briquettes, sorbent |
| Bio-oil (Liquid) | Condensed liquid from vapors | Industrial fuel, refined transportation fuels, chemicals |
| Syngas (Gas) | Non-condensable combustible gases | Powering the pyrolysis process, heat/power generation |
Ready to harness the power of pyrolysis for your lab or facility?
KINTEK specializes in advanced laboratory equipment and consumables for research and process development in thermal conversion technologies like pyrolysis. Whether you're exploring feedstock suitability, optimizing process conditions, or analyzing product yields, our reliable equipment provides the precision and control you need.
We help our customers:
- Accelerate R&D with precise, bench-scale pyrolysis systems.
- Optimize Processes by accurately controlling temperature and residence time.
- Analyze Products with equipment designed for characterizing bio-oil, bio-char, and syngas.
Transform your waste streams into valuable resources. Contact KINTEK today to discuss your specific laboratory needs and discover the right equipment for your pyrolysis projects.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Lab-Scale Vacuum Induction Melting Furnace
People Also Ask
- What are the different types of pyrolysis machines? Choose the Right System for Your Output
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds
- What are the reactions involved in pyrolysis of biomass? Unlock the Chemistry for Tailored Bio-Products
- What are the components of biomass pyrolysis? A Complete Guide to the System, Products, and Process
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions



















