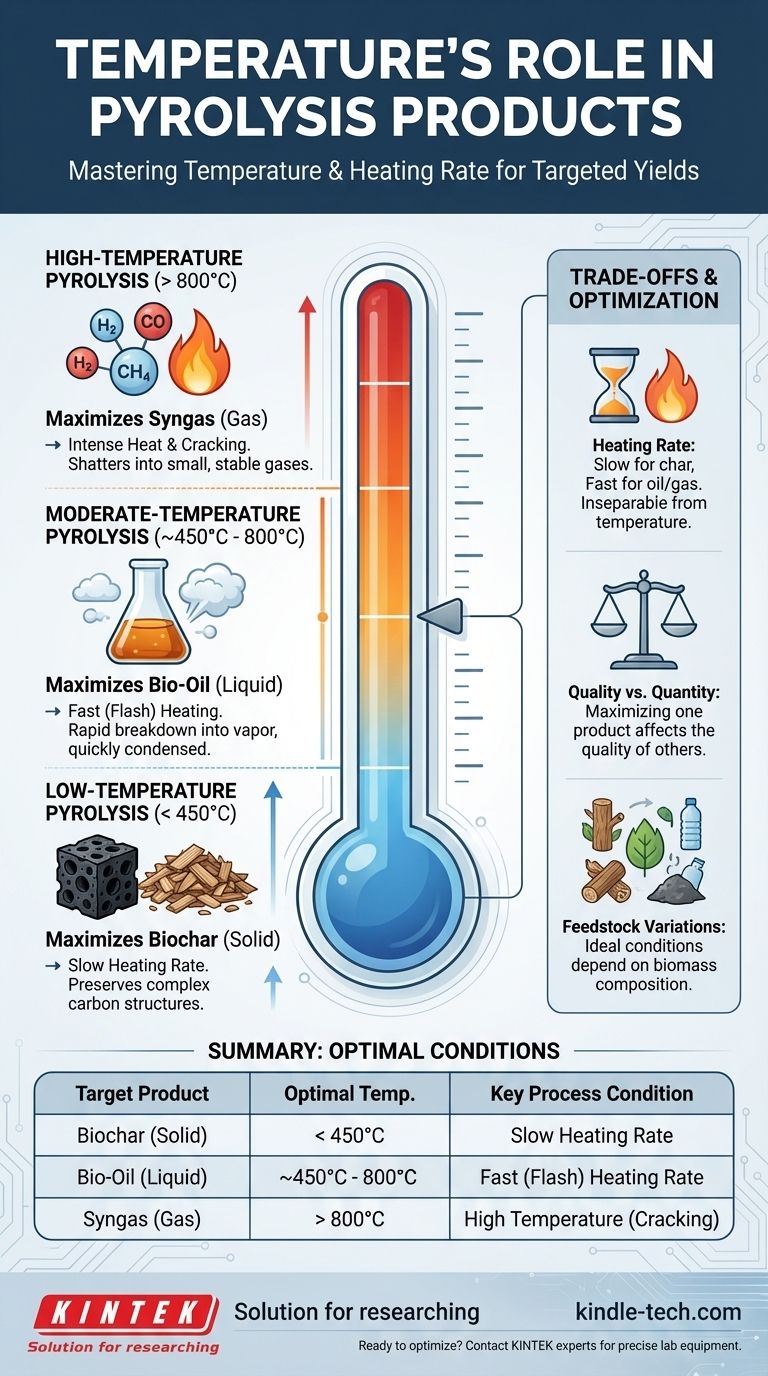
In pyrolysis, temperature is the primary control lever that dictates the final product distribution. In short, lower temperatures favor the production of solid biochar, high temperatures maximize gas yield, and intermediate temperatures are used to create liquid bio-oil. The rate at which the target temperature is reached is also a critical factor in determining the outcome.
To effectively control pyrolysis, you must understand that temperature and heating rate are not just process settings; they are tools for selectively breaking down biomass. Low and slow conditions preserve complex carbon structures (char), while high and fast conditions shatter them into simple gases, with the valuable liquid oils found in between.

How Temperature Governs Pyrolysis Yields
Temperature directly influences which chemical bonds within the biomass feedstock break and how the resulting smaller molecules react. Each product—solid, liquid, or gas—has an optimal thermal window for its formation.
Low-Temperature Pyrolysis (< 450°C)
At lower temperatures, typically combined with slow heating rates, the process primarily drives off water and the most volatile organic compounds.
This gentle thermal decomposition preserves the underlying carbon backbone of the material. The result is a maximized yield of biochar, a stable, carbon-rich solid.
Moderate-Temperature Pyrolysis (~450°C - 800°C)
This range, especially when paired with very high heating rates, is the domain of fast pyrolysis.
The rapid energy input breaks down larger polymers like cellulose and lignin into smaller, vaporized molecules. These vapors are then quickly cooled and condensed, preventing them from breaking down further into gases. This process maximizes the yield of liquid bio-oil.
High-Temperature Pyrolysis (> 800°C)
At very high temperatures, the thermal energy is so intense that it causes extensive "cracking." Not only do the original biomass structures break down, but so do the intermediate vapor and oil molecules.
This secondary cracking shatters everything into the smallest, most stable gaseous molecules, such as hydrogen, carbon monoxide, and methane. The primary product is therefore syngas (synthesis gas).
Understanding the Trade-offs
Simply choosing a temperature is not enough. The efficiency and quality of your desired product depend on balancing several interconnected factors.
The Interplay of Temperature and Heating Rate
Temperature and heating rate are inseparable. A slow ramp-up to 800°C will produce a very different result than a near-instantaneous flash heating to the same temperature.
Slow heating allows time for solid char to form and stabilize. Rapid heating "outruns" char formation, quickly converting the biomass into vapors essential for oil or gas production.
Product Quantity vs. Quality
Optimizing for the highest possible yield of one product fraction can impact its quality. For instance, fast pyrolysis aimed at maximum bio-oil yield might produce a more acidic or unstable oil if not managed correctly.
Likewise, pushing for maximum gas yield comes at the complete expense of any significant char or oil output. The choice of one product inherently means sacrificing the others.
Feedstock Variations
The ideal temperature ranges are not fixed for all materials. The specific composition of the biomass feedstock (e.g., wood, agricultural waste, plastics) will influence the optimal conditions.
Different materials contain varying ratios of cellulose, hemicellulose, and lignin, each of which decomposes at a slightly different temperature, shifting the ideal processing window.
Optimizing Temperature for Your Goal
To apply this knowledge effectively, align your process parameters with your desired final product.
- If your primary focus is carbon sequestration or soil amendment: Use slow pyrolysis at temperatures below 450°C to maximize the yield of stable, solid biochar.
- If your primary focus is creating a liquid biofuel or chemical feedstock: Use fast pyrolysis at moderate temperatures (around 500-750°C) with rapid heating to maximize bio-oil yield.
- If your primary focus is generating energy or synthesis gas (syngas): Use high-temperature pyrolysis (above 800°C) to maximize the conversion of biomass into combustible gases.
By mastering temperature and heating rate, you transform pyrolysis from a simple decomposition process into a precise tool for creating value.
Summary Table:
| Target Product | Optimal Temperature Range | Key Process Condition |
|---|---|---|
| Biochar (Solid) | < 450°C | Slow Heating Rate |
| Bio-Oil (Liquid) | ~450°C - 800°C | Fast (Flash) Heating Rate |
| Syngas (Gas) | > 800°C | High Temperature (Cracking) |
Ready to optimize your pyrolysis process for maximum yield?
At KINTEK, we specialize in providing the precise lab equipment and consumables you need to master thermal processes like pyrolysis. Whether your goal is to maximize biochar for carbon sequestration, produce high-quality bio-oil for fuel, or generate syngas for energy, our reactors and temperature control systems are engineered for reliability and repeatability.
We help you:
- Achieve precise temperature control and rapid heating rates.
- Select the right equipment for your specific feedstock and target product.
- Improve the efficiency and quality of your pyrolysis outputs.
Let's discuss your application. Contact our experts today to find the perfect solution for your laboratory's pyrolysis needs.
Visual Guide
Related Products
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What is the range of pyrolysis? Master Temperature Control for Optimal Bio-Product Yields
- How do high-temperature reaction furnaces control in-situ MMCs? Master Material Precision and Structural Integrity
- What are the main types of biomass conversion processes? Unlock the Best Pathway for Your Energy Needs
- What are the process advantages of using a rotary tube furnace for WS2 powder? Achieve Superior Material Crystallinity
- What temperature is needed for pyrolysis waste? A Guide to Optimizing Your Waste-to-Value Process



















