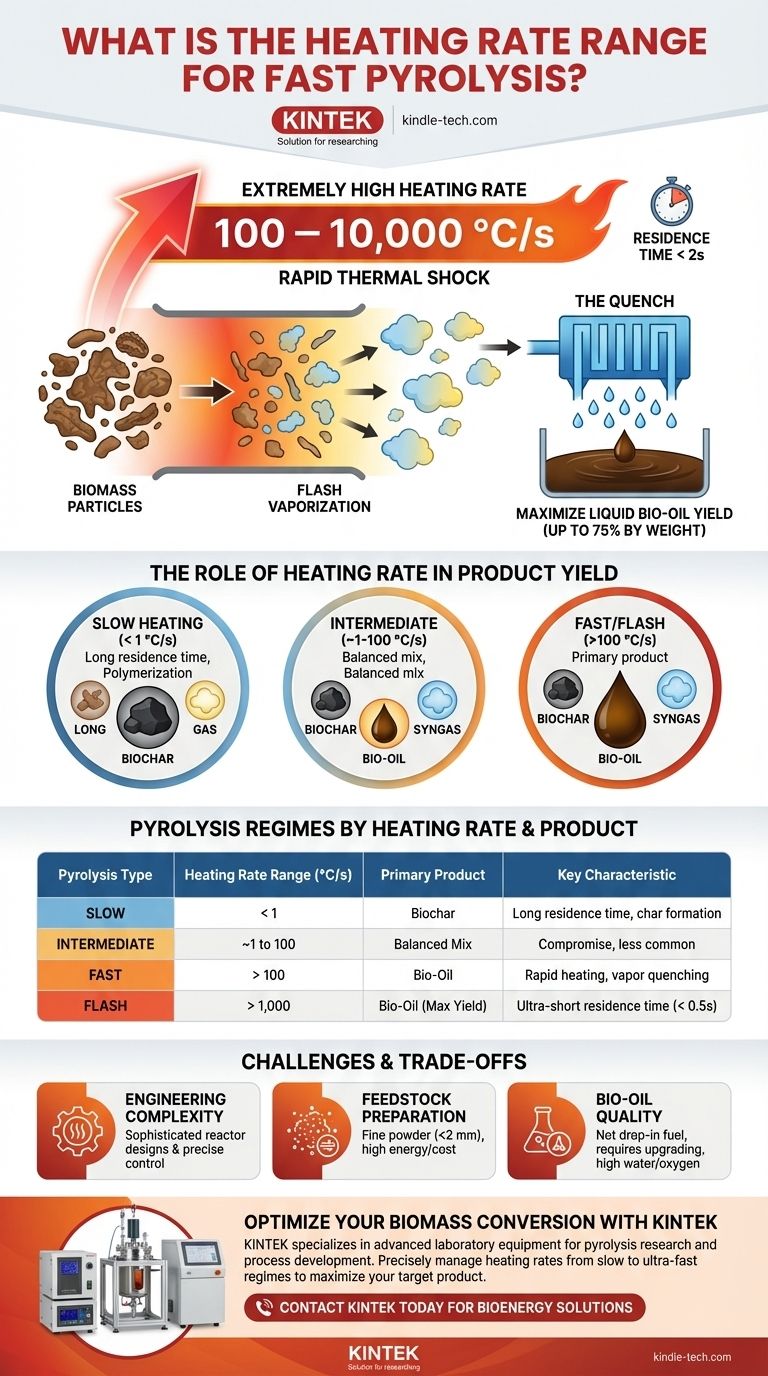
In fast pyrolysis, the required heating rate is extremely high, typically ranging from 100 to 10,000 °C per second (°C/s), and in some systems, even higher. This rapid thermal shock is the defining characteristic of the process and is intentionally designed to maximize the production of liquid bio-oil from biomass.
The core principle of fast pyrolysis is to heat biomass so quickly that its constituent polymers (cellulose, hemicellulose, lignin) fracture into vapor fragments and are removed from the hot zone before they can undergo secondary reactions that would otherwise form more char and gas.

The Role of Heating Rate in Product Yield
The heating rate is arguably the most critical parameter in any pyrolysis process. It directly controls the reaction pathways and, therefore, the final distribution of the three primary products: solid biochar, liquid bio-oil, and non-condensable syngas.
Suppressing Secondary Reactions
At slow heating rates, the initial breakdown of biomass creates primary vapors. These vapors have a long time to linger in the hot reactor, where they polymerize on the surface of the solid char or crack into smaller, lower-molecular-weight gas molecules. This is why slow pyrolysis yields a large amount of biochar.
Fast pyrolysis defeats this mechanism. The extreme heating rate provides so much energy so quickly that the biomass is essentially "flash-vaporized," minimizing the time available for these secondary char- and gas-forming reactions.
Maximizing Primary Vapors
The goal of fast pyrolysis is to shear the long polymer chains of biomass into smaller, condensable organic molecules. High heating rates favor these primary decomposition reactions, creating a large volume of vapors and aerosols.
This rapid generation of vapors is immediately followed by a very short vapor residence time (typically less than 2 seconds), where the products are quickly removed from the reactor.
The "Quench" is Equally Critical
Achieving a high heating rate is only half the battle. To preserve the valuable liquid compounds, these hot vapors must be cooled, or "quenched," just as rapidly.
This rapid cooling condenses the vapors into a liquid—the bio-oil—before they have a chance to thermally crack into non-condensable gases. The combination of rapid heating and rapid quenching is what allows for bio-oil yields of up to 75% by weight.
Comparing Pyrolysis Regimes by Heating Rate
Understanding the spectrum of pyrolysis helps to contextualize the extreme nature of the fast pyrolysis heating rate.
Slow Pyrolysis: < 1 °C/s
This is a very slow, controlled heating process that can take minutes to hours. The primary goal here is to maximize the production of biochar, a stable, carbon-rich solid. The long residence time encourages secondary reactions that build the char matrix.
Intermediate Pyrolysis: ~1 to 100 °C/s
Occupying the middle ground, intermediate pyrolysis produces a more balanced slate of biochar, bio-oil, and syngas. It is less common in commercial applications, which typically optimize for either char or oil.
Fast & Flash Pyrolysis: > 100 °C/s
This regime is defined by its focus on producing bio-oil. The term "flash pyrolysis" is often used to describe the upper end of this spectrum (> 1,000 °C/s) with even shorter vapor residence times (< 0.5 seconds), further emphasizing the goal of maximizing liquid yield.
Understanding the Trade-offs and Challenges
While fast pyrolysis is effective at producing bio-oil, its demanding process conditions come with significant challenges.
Engineering Complexity
Achieving heat transfer rates of over 100 °C/s is not trivial. It requires sophisticated reactor designs, such as circulating fluidized bed or ablative reactors, and very fine control over a high-temperature process.
Feedstock Preparation Requirements
To heat a particle rapidly, it must be very small. Biomass feedstock for fast pyrolysis must be thoroughly dried and ground to a fine powder (typically < 2 mm). This pre-processing adds significant energy and cost to the overall operation.
Bio-oil Quality
The resulting raw bio-oil is not a drop-in replacement for fossil fuels. It is acidic, contains a high percentage of water (15-30%), is thermally unstable, and has a high oxygen content. It requires significant and costly upgrading to be used as a transportation fuel.
Making the Right Choice for Your Goal
The ideal heating rate is not a universal constant; it is dictated entirely by your desired primary product.
- If your primary focus is producing biochar: Opt for slow pyrolysis with heating rates below 1 °C/s to maximize solid yield and carbon stability.
- If your primary focus is maximizing liquid bio-oil: You must use fast pyrolysis with heating rates exceeding 100 °C/s, coupled with rapid vapor quenching.
- If your primary focus is a more balanced distribution of products or simpler reactor design: Intermediate pyrolysis may offer a workable compromise, though it is not optimized for any single product.
Ultimately, mastering the heating rate is the key to directing biomass conversion toward your intended outcome.
Summary Table:
| Pyrolysis Type | Heating Rate Range (°C/s) | Primary Product | Key Characteristic |
|---|---|---|---|
| Slow Pyrolysis | < 1 | Biochar | Long residence time for char formation |
| Intermediate Pyrolysis | ~1 to 100 | Balanced Mix | Compromise between char, oil, and gas |
| Fast Pyrolysis | > 100 | Bio-Oil | Rapid heating and vapor quenching |
| Flash Pyrolysis | > 1,000 | Bio-Oil (Max Yield) | Ultra-short vapor residence time (< 0.5s) |
Ready to optimize your biomass conversion process? The right heating rate is critical for achieving your target product yields. KINTEK specializes in advanced laboratory equipment for pyrolysis research and process development. Our reactors and temperature control systems help you precisely manage heating rates from slow to ultra-fast flash pyrolysis regimes. Whether you're focused on maximizing bio-oil, biochar, or syngas production, our experts can provide the equipment and support you need to scale your technology from lab to pilot plant. Contact our team today to discuss your specific application and discover how KINTEK's solutions can accelerate your bioenergy and bioproduct development.
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Multi-zone Laboratory Tube Furnace
- Molybdenum Disilicide (MoSi2) Thermal Elements Electric Furnace Heating Element
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
People Also Ask
- What are the products of pyrolysis of biomass? Unlock Bio-Char, Bio-Oil, and Syngas
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What are the conditions for biomass pyrolysis? Optimize Temperature, Heating Rate & Time
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions
- What are the different types of pyrolysis machines? Choose the Right System for Your Output



















