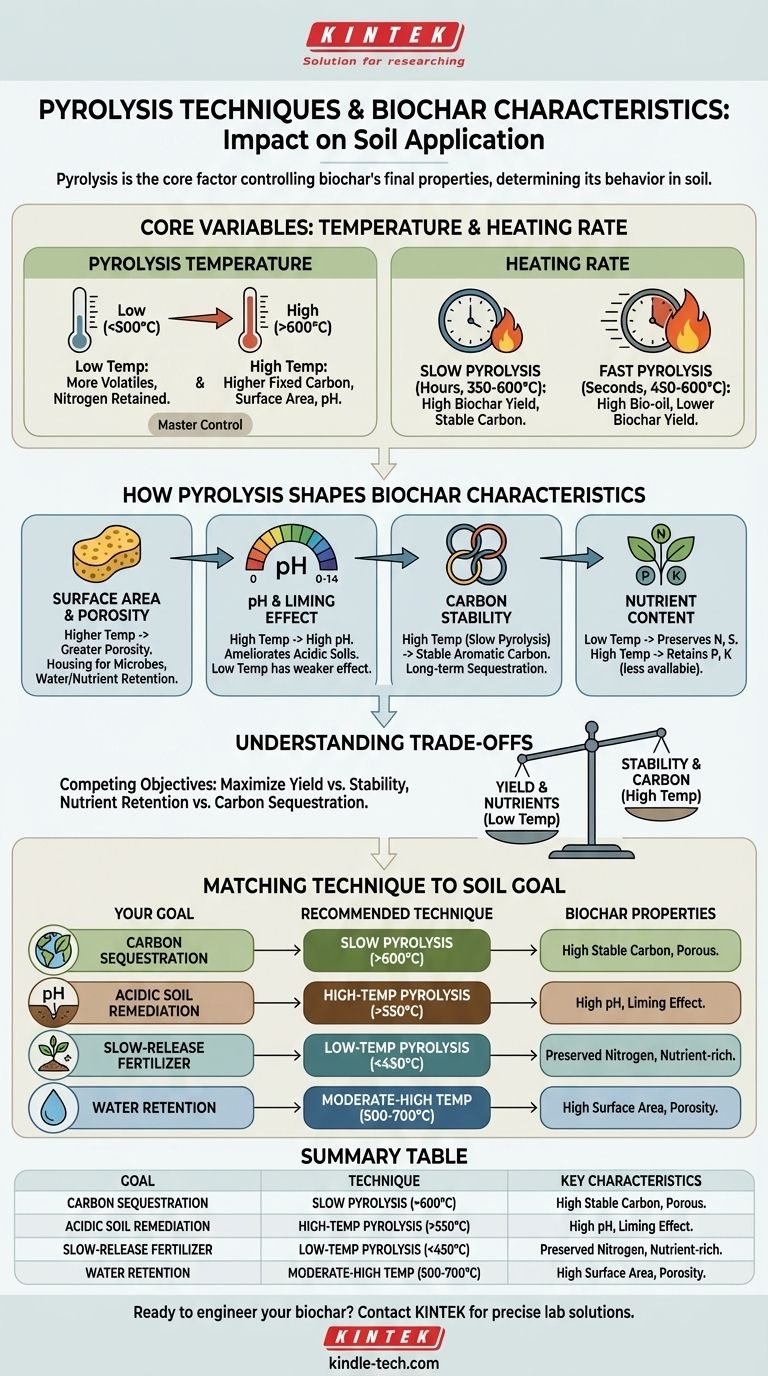
At its core, the pyrolysis technique is the single most important factor controlling the final characteristics of biochar. The specific temperature and heating rate used during production directly determine the biochar's pH, porosity, surface area, and nutrient stability, which in turn dictate its behavior and effectiveness once applied to the soil.
The choice of pyrolysis method is a strategic decision based on trade-offs. Slow pyrolysis at high temperatures creates a stable, high-carbon biochar ideal for carbon sequestration, while lower temperatures preserve more nutrients. Understanding this relationship is key to engineering a biochar for a specific agricultural or environmental goal.

The Core Variables: Temperature and Heating Rate
The thermochemical conversion of biomass into biochar is governed by two primary levers: the final temperature reached and the rate at which that temperature is achieved.
Pyrolysis Temperature: The Master Control Variable
The peak temperature during pyrolysis is the dominant factor influencing biochar's final properties. Lower temperatures (< 500°C) result in a higher yield of char that retains more volatile organic compounds and nutrients like nitrogen.
Conversely, higher temperatures (> 600°C) drive off more volatiles, creating a char with higher fixed carbon content, greater surface area, increased pH, and more stable aromatic carbon structures.
Heating Rate: Slow vs. Fast Pyrolysis
The heating rate distinguishes the two main modes of pyrolysis. Slow pyrolysis involves heating biomass slowly (e.g., 5-30°C per minute) to moderate temperatures (350-600°C) with a long residence time (hours).
Fast pyrolysis, in contrast, involves heating biomass extremely rapidly (hundreds or thousands of degrees per second) to moderate temperatures (450-600°C) with a very short residence time (seconds).
How Pyrolysis Shapes Key Biochar Characteristics
Each production parameter directly translates into a physical or chemical property relevant to soil health.
Surface Area and Porosity
Higher pyrolysis temperatures create greater surface area and porosity. As temperature increases, volatile matter is driven out of the biomass structure, leaving behind a network of micropores and macropores.
This porous structure is critical for biochar's function in soil, as it provides housing for beneficial microbes and enhances the soil's capacity to retain water and dissolved nutrients.
pH and Liming Effect
Biochar produced at high temperatures typically has a high pH. This occurs because acidic functional groups on the char's surface are thermally decomposed, while inorganic alkali salts (potassium, calcium, magnesium) from the original feedstock become concentrated.
This gives high-temperature biochar a significant liming effect, making it highly effective for ameliorating acidic soils. Low-temperature chars have a much weaker effect on soil pH.
Carbon Stability and Sequestration Potential
The primary goal of carbon sequestration is to lock carbon into a form that resists decomposition for centuries. This requires highly stable carbon.
Slow pyrolysis at high temperatures (>600°C) is most effective for this, as it promotes the formation of fused aromatic ring structures similar to graphite. Biochar produced at lower temperatures contains less stable forms of carbon that can be more easily mineralized by soil microbes.
Nutrient Content and Availability
There is a direct trade-off between pyrolysis temperature and nutrient retention. Volatile nutrients like nitrogen (N) and sulfur (S) are largely lost at temperatures above 500°C.
To create a biochar intended as a nutrient source, a lower-temperature process (< 450°C) is required. Mineral nutrients like phosphorus (P) and potassium (K) are retained at high temperatures, but their bioavailability can sometimes decrease as they become embedded in stable crystalline structures.
Understanding the Trade-offs
Producing biochar is not about finding a single "best" method; it's about managing competing objectives.
The Yield vs. Stability Dilemma
Slow pyrolysis maximizes the yield of solid biochar (up to 35% by weight), making it efficient for producing a soil amendment. However, achieving maximum carbon stability requires high temperatures, which slightly reduces the overall mass yield.
Fast pyrolysis, on the other hand, is optimized to produce liquid bio-oil (up to 75% by weight), with biochar being a lower-yield co-product (around 12%).
Nutrient Retention vs. Carbon Sequestration
The goals of maximizing nutrient retention and maximizing carbon stability are fundamentally in conflict. The low temperatures needed to preserve nitrogen result in a less stable carbon structure. The high temperatures needed for stable carbon will volatilize most of the available nitrogen.
The Feedstock Factor
The pyrolysis process modifies the feedstock; it does not create properties from scratch. A feedstock rich in minerals, like manure or biosolids, will inherently produce a high-ash, high-pH biochar rich in P and K. A woody feedstock will produce a low-ash, high-carbon biochar. The pyrolysis technique refines these inherent tendencies.
Matching Pyrolysis Technique to Your Soil Goal
The optimal biochar is not universal; it is defined by your specific objective. When choosing or producing biochar, consider your primary goal.
- If your primary focus is long-term carbon sequestration and improving soil structure: Choose a biochar made via slow pyrolysis at high temperatures (>600°C) from a woody feedstock to maximize stable, aromatic carbon content.
- If your primary focus is remediating acidic soils: Choose a biochar produced at a high temperature (>550°C) to ensure a high pH and strong liming capacity.
- If your primary focus is creating a slow-release fertilizer: Choose a biochar made from a nutrient-rich feedstock (e.g., manure) via low-temperature pyrolysis (<450°C) to preserve volatile nutrients like nitrogen.
- If your primary focus is improving soil water retention: Choose a biochar produced at moderate-to-high temperatures (500-700°C) to maximize the development of porous surface area.
By understanding the relationship between pyrolysis conditions and biochar properties, you can precisely engineer a soil amendment for your desired outcome.
Summary Table:
| Pyrolysis Goal | Recommended Technique | Key Biochar Characteristics |
|---|---|---|
| Carbon Sequestration | Slow Pyrolysis (>600°C) | High stable carbon, porous structure |
| Acidic Soil Remediation | High-Temp Pyrolysis (>550°C) | High pH, strong liming effect |
| Slow-Release Fertilizer | Low-Temp Pyrolysis (<450°C) | Preserved nitrogen, nutrient-rich |
| Water Retention | Moderate-High Temp (500-700°C) | High surface area, porosity |
Ready to engineer the perfect biochar for your soil? At KINTEK, we specialize in providing the precise lab equipment and consumables needed to optimize your pyrolysis process. Whether you're researching carbon sequestration, soil remediation, or nutrient management, our solutions help you achieve consistent, high-quality results. Contact our experts today to discuss how we can support your laboratory's biochar and soil science projects!
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Pyrolysis Furnace Plant Machine Calciner Small Rotary Kiln Rotating Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Electric Rotary Kiln Small Rotary Furnace for Activated Carbon Regeneration
People Also Ask
- What are the components of biomass pyrolysis? A Complete Guide to the System, Products, and Process
- How is energy converted into biomass? Harnessing Nature's Solar Power for Renewable Energy
- What is the process of biomass fast pyrolysis? Turn Biomass into Bio-Oil in Seconds
- What are the different types of pyrolysis machines? Choose the Right System for Your Output
- What are the advantages of pyrolysis technology? Turn Waste into Profit and Reduce Emissions



















