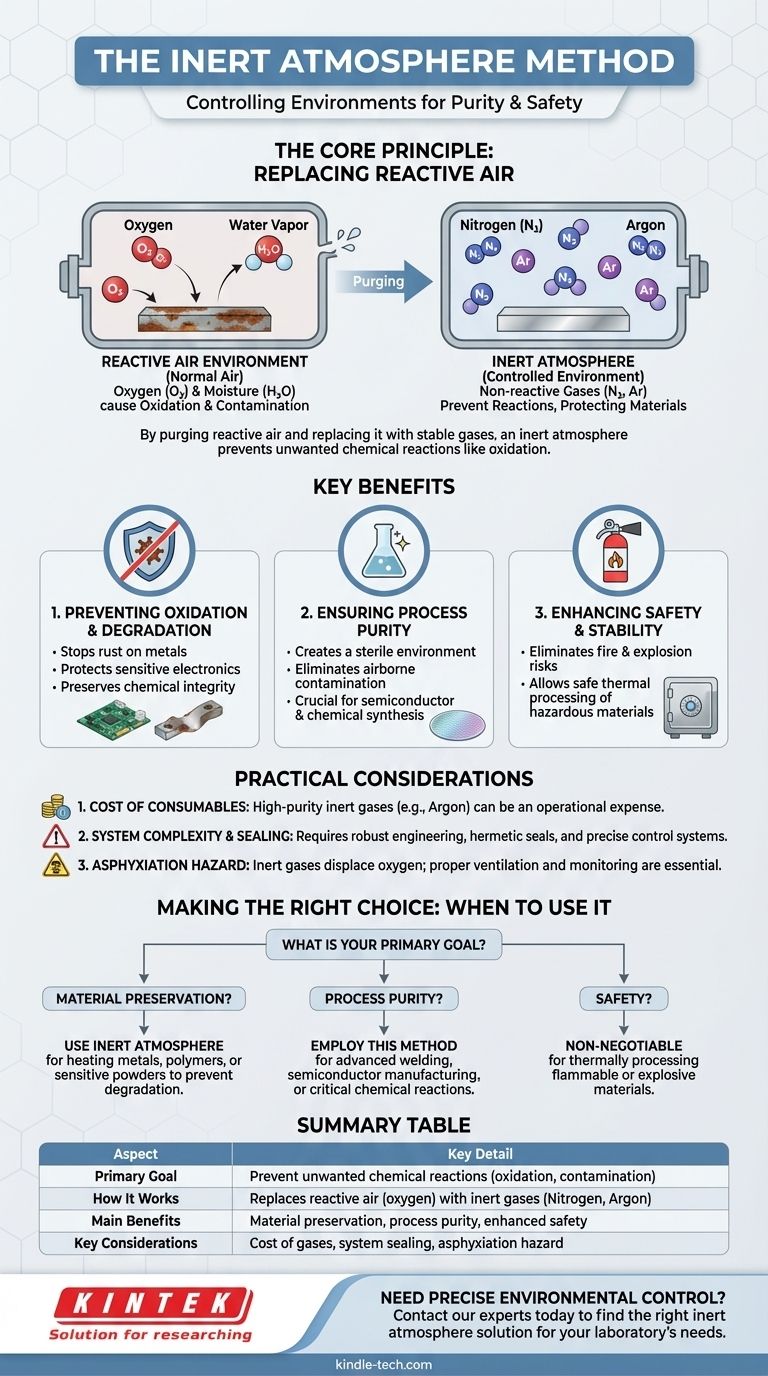
In essence, the inert atmosphere method is a technique used to control the environment within a sealed space by replacing the normal, reactive air with a non-reactive, or "inert," gas. This process is crucial for preventing unwanted chemical reactions, primarily oxidation and contamination, that would otherwise occur in the presence of oxygen and moisture.
The fundamental goal of the inert atmosphere method is to protect a sensitive material or process from degradation by removing reactive elements like oxygen and creating a stable, non-reactive environment.

The Core Principle: Replacing Reactive Air
Why Normal Air is a Problem
The air we breathe is approximately 78% nitrogen, 21% oxygen, and has traces of other gases and water vapor. While essential for life, oxygen is highly reactive.
When heated or exposed to certain materials, oxygen causes oxidation (like rust on iron) and can trigger other undesirable chemical reactions. This can degrade material quality, compromise chemical purity, or even create safety hazards.
How Inert Gases Provide a Solution
The inert atmosphere method works by purging the reactive air from a sealed chamber, like an oven or a glove box, and replacing it with a stable gas.
Gases like nitrogen and argon are commonly used because they are chemically inert. They do not readily react with other substances, even under high temperatures, which makes them ideal protective blankets for sensitive processes.
Key Benefits of an Inert Atmosphere
Preventing Oxidation and Degradation
The most common application is to prevent materials from breaking down. By removing oxygen, this method stops the oxidation of metals, protects sensitive electronic components during soldering, and preserves the integrity of chemical powders during heat treatment.
Ensuring Process Purity
In high-precision fields like electronics manufacturing or chemical synthesis, even minuscule contamination from airborne particles or side reactions with oxygen can ruin a product.
An inert atmosphere creates a sterile environment, ensuring that the only reactions that occur are the ones you intend.
Enhancing Safety and Stability
Many materials can become flammable or even explosive when heated in the presence of oxygen.
By creating an oxygen-free environment, the inert atmosphere method eliminates the risk of fire and explosions, allowing for the safe thermal processing of otherwise hazardous materials.
Understanding the Practical Considerations
Cost of Consumables
The primary drawback is the ongoing cost of the inert gas itself. While nitrogen is relatively inexpensive, high-purity argon can be a significant operational expense, especially for large-scale or continuous operations.
System Complexity and Sealing
Implementing this method requires specialized equipment. The chamber must be hermetically sealed to prevent ambient air from leaking back in. This requires robust engineering, high-quality seals, and precise control systems to manage gas flow, which increases initial setup costs.
Asphyxiation Hazard
A critical safety consideration is the risk of asphyxiation. Inert gases displace oxygen. In the event of a significant leak into a poorly ventilated room, they can create an environment that cannot support life. Proper ventilation and oxygen monitoring are essential safety protocols.
Making the Right Choice for Your Goal
To determine if this method is right for you, consider your primary objective.
- If your primary focus is material preservation: Use an inert atmosphere to prevent oxidation and thermal degradation when heating metals, polymers, or sensitive powders.
- If your primary focus is process purity: Employ this method for advanced welding, semiconductor manufacturing, or chemical reactions where atmospheric contamination is unacceptable.
- If your primary focus is safety: This method is non-negotiable when thermally processing materials that are flammable or explosive in the presence of oxygen.
Ultimately, the inert atmosphere method is a powerful tool for achieving control, purity, and safety in sensitive technical processes.
Summary Table:
| Aspect | Key Detail |
|---|---|
| Primary Goal | Prevent unwanted chemical reactions (oxidation, contamination) |
| How It Works | Replaces reactive air (oxygen) with inert gases (Nitrogen, Argon) |
| Main Benefits | Material preservation, process purity, enhanced safety |
| Key Considerations | Cost of gases, system sealing, asphyxiation hazard |
Need precise environmental control for your lab processes?
KINTEK specializes in providing the lab equipment and consumables, including inert atmosphere ovens and gas systems, to protect your sensitive materials and ensure process purity. Our solutions help you prevent oxidation, eliminate contamination, and operate safely.
Contact our experts today to find the right inert atmosphere solution for your laboratory's needs.
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the difference between oxidizing and reducing atmospheres? Key Insights for Your Applications
- What gases are used in brazing? Optimize Your Brazing Process with the Right Atmosphere
- What is an inert or reducing atmosphere? Master Process Control for Your Lab
- Why is argon used when an inert atmosphere is needed? The Ultimate Guide to Chemical Stability
- What is the brazing process? A Guide to Strong, Versatile, and Aesthetic Joining
- Why is high-purity argon protection essential for titanium dioxide reduction? Ensure Peak Metal Purity
- Why are inert gases crucial in brazing? Protect Joints and Enhance Quality with Nitrogen, Helium, and Argon
- How can an atmosphere furnace be used to control the surface carbonate content in Li2ZrO3-coated NCM622 materials?



















