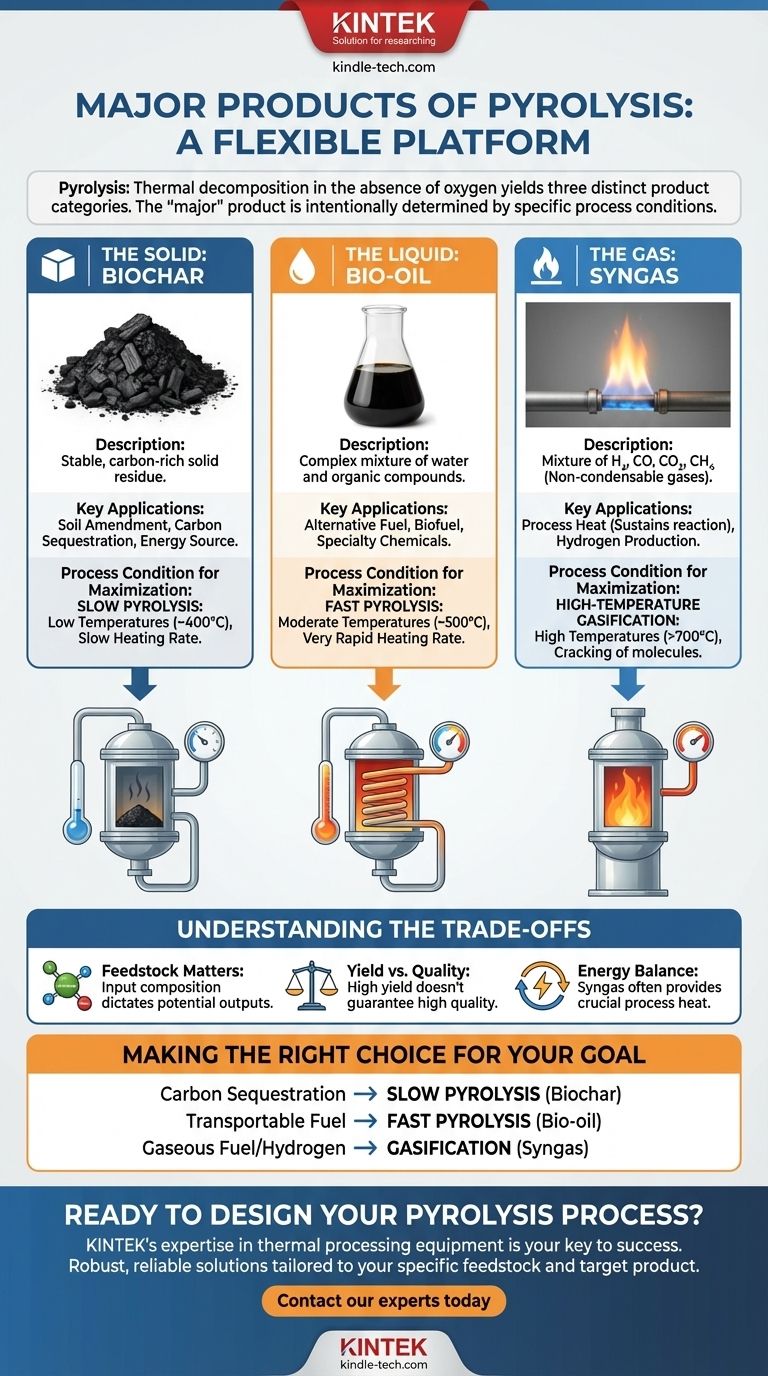
The major products of pyrolysis fall into three distinct categories: a solid, a liquid, and a gas. Specifically, the thermal decomposition of a material like biomass in the absence of oxygen yields biochar (the solid), bio-oil (the liquid), and syngas (the non-condensable gas). Which of these is considered the "major" product is not fixed; it is intentionally determined by the specific process conditions used.
The central takeaway is that pyrolysis is not a single process but a flexible platform. The "major" product is a direct result of tuning variables like temperature and heating rate to maximize the yield of either the solid (biochar), liquid (bio-oil), or gas (syngas) based on the desired outcome.

The Three Core Products of Pyrolysis
Pyrolysis breaks down complex organic materials into simpler, more valuable components. Regardless of the specific setup, the output can be consistently grouped into three primary states of matter.
The Solid: Biochar
Biochar is a stable, carbon-rich solid that is often visually similar to charcoal. It is the solid residue left after volatile components have been driven off the original feedstock.
Its primary applications include soil amendment in agriculture to improve fertility and water retention, as well as for carbon sequestration. It can also be used as an energy source or processed into activated carbon.
The Liquid: Bio-oil
Also known as pyrolysis oil or tar, bio-oil is a complex mixture of water, organic acids, alcohols, and hundreds of other organic compounds. It is the result of condensing the volatile gases produced during the reaction.
This dense liquid can be burned as an alternative fuel for heat and power generation or refined into higher-value biofuels and specialty chemicals. Its high energy density makes it easier to transport than raw biomass.
The Gas: Syngas
Syngas, or synthesis gas, is the stream of non-condensable gases that remain after the bio-oil has been separated. It is primarily a mixture of hydrogen, carbon monoxide, carbon dioxide, and methane.
While it can be collected, the most common use for syngas is to be cycled back into the pyrolysis plant to provide the heat energy needed to sustain the reaction, making the process more efficient.
How Process Conditions Determine the "Major" Product
The distribution of these three products is not random. It is a direct consequence of the process parameters. By controlling these variables, operators can effectively choose their desired primary output.
Slow Pyrolysis (Maximizing Biochar)
To maximize the yield of biochar, a slow pyrolysis process is used. This involves relatively low temperatures (around 400°C) and a slow heating rate. These conditions allow the carbon in the feedstock to stabilize into a solid structure rather than breaking down into volatile gases.
Fast Pyrolysis (Maximizing Bio-oil)
To maximize the yield of bio-oil, a fast pyrolysis process is essential. This requires moderate temperatures (around 500°C) and a very rapid heating rate. The biomass must be heated so quickly that it vaporizes before significant charring can occur, and these vapors are then rapidly cooled to form the liquid oil.
Gasification (Maximizing Syngas)
To maximize the yield of syngas, the process is pushed towards gasification. This involves high temperatures (typically >700°C), which crack the heavier molecules, including the tars that would form bio-oil, into the simplest gaseous components like hydrogen and carbon monoxide.
Understanding the Trade-offs
Choosing a target product involves navigating key technical and practical considerations. The ideal process is rarely the simplest.
The Feedstock Matters
The starting material, or feedstock, has a profound impact on the output. Pyrolysis of biomass (containing carbon, hydrogen, and oxygen) yields bio-oil and the other products described.
However, the pyrolysis of a different feedstock, like methane gas (CH4), will yield entirely different products: solid carbon and gaseous hydrogen. This illustrates how the chemical composition of the input material dictates the potential outputs.
Yield vs. Quality
Maximizing the yield of a specific product does not guarantee its quality. For example, while fast pyrolysis can produce a high volume of bio-oil, this oil is often acidic, unstable, and requires significant upgrading or refining before it can be used as a direct replacement for conventional fuels.
Energy Balance
A pyrolysis system must be energy efficient to be viable. While syngas has value, its most critical role is often providing the energy to run the reactor. A process that produces too little gas may require an external energy source, increasing operational costs and complexity.
Making the Right Choice for Your Goal
The "major" product of pyrolysis is the one you design the process to create. Your decision should be guided by your end goal.
- If your primary focus is carbon sequestration or soil improvement: You will use slow pyrolysis to maximize the yield of stable biochar.
- If your primary focus is creating a transportable liquid fuel: You will use fast pyrolysis to maximize the yield of bio-oil.
- If your primary focus is generating gaseous fuel or hydrogen: You will use high-temperature gasification to maximize the yield of syngas.
Ultimately, pyrolysis is best understood as a versatile conversion technology that transforms low-value materials into a tailored slate of higher-value products.
Summary Table:
| Product Type | Primary Output | Key Process Conditions | Common Applications |
|---|---|---|---|
| Solid | Biochar | Slow Pyrolysis (Low Temp, Slow Heating) | Soil Amendment, Carbon Sequestration |
| Liquid | Bio-oil | Fast Pyrolysis (Moderate Temp, Rapid Heating) | Alternative Fuel, Chemical Feedstock |
| Gas | Syngas | High-Temperature Pyrolysis/Gasification | Process Heat, Hydrogen Production |
Ready to design your pyrolysis process?
Whether your goal is to maximize biochar for carbon sequestration, produce bio-oil for energy, or generate syngas, KINTEK's expertise in thermal processing equipment is your key to success. We provide robust, reliable solutions tailored to your specific feedstock and target product.
Let's build your ideal pyrolysis system together. Contact our experts today to discuss your project!
Visual Guide
Related Products
- Customizable Laboratory High Temperature High Pressure Reactors for Diverse Scientific Applications
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- High Temperature Constant Temperature Heating Circulator Water Bath Chiller Circulator for Reaction Bath
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Wall Mounted Water Distillation Unit
People Also Ask
- What is the process of pyrolysis waste management? Turn Waste into Valuable Resources
- What are the advantages and disadvantages of flash pyrolysis? Maximize Bio-Oil Yield vs. High Costs
- What is the heat required for calcination? A Guide to Accurate Energy Calculations
- What equipment is used for calcination? Choosing the Right System for Your Process
- What are the feedstocks for pyrolysis? Unlock the Potential of Organic Materials
- What are the three types of pyrolysis process? Slow, Fast, and Conventional Explained
- What is the significance of calcination? A Guide to Purification and Metal Extraction
- How do you make biochar pyrolysis? A Guide to Converting Biomass into Stable Carbon



















